Chemistry 1st term

Chemistry Quiz: Test Your Knowledge!
Welcome to the Chemistry 1st Term Quiz! This engaging quiz consists of 17 questions designed to challenge your understanding of fundamental chemistry concepts. Test your knowledge and see how well you can answer questions related to chemical systems, enthalpy, and more!
Quiz Features:
- 17 thought-provoking questions
- Multiple choice answers for each question
- Instant feedback upon completion
1) The Egg is considered an example of .............. system
A) Closed
B) Open
C) Closed or open
D) isolated
2) The combustion enthalphy of different substances is accurately measured by using .....
A) Glass calorimeter
B) Bomb calorimeter
C) thermo meter
D) Manometer
4) N , M and L are 3 electrons of mass numbers equal 235 , 238 and 239 respectively . If you know that the atom of element L has 92 electrons and the atom of element M has 92 protons and the atom of element N has 145 electrons . Which of them are isotopes?
A) L and M only
B) L and N only
C) M and N only
D) L , M and N
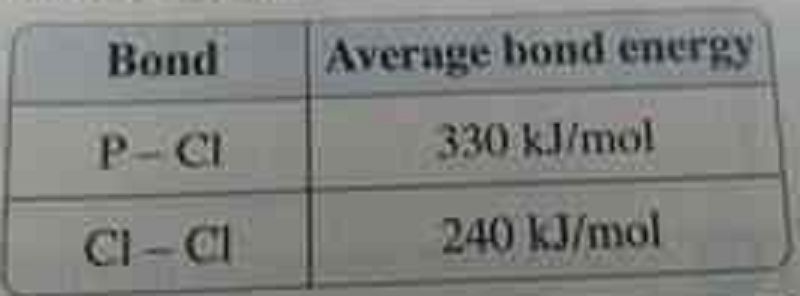
5) Phosphorus pentachloride gas decomposes by hear to phosphorus trichloride gas and chlorine gas , what is the amount of change in the heat content of this reaction ?
A) -90 KJ/Mol
B) +90 KJ/Mol
C) -420 KJ/Mol
D) +420 KJ/mol

7 )
AlCl3 < Al(OH)3 < Al2(SO4)3
Al2(SO4)3 < Al(OH)3 < Alcl3
Al(OH)3 < Al2(SO4)3 < AlCL3
Al(OH)3 < Alcl3 < Al2(SO4)3
8) The change in heat content can be measured by using
A) Hess law only
B) The calorimeter only
C) Hess's law or the calorimeter
D) the thermometer
9 ) Which of the following systems does not permit the transferring of energy outside the system boundaries
A) the closed systems
B) the open system
C) the special system
D) The isolated system

10 )
A) The quantity of heat produced from the combustion of 1 g of octane = 96 KJ
B) The quantity of heat produced from the combustion of 1 g of methanol = 22.65 KJ
C) The quantity of heat produced from the combustion of 1 Kg of octane = 9 times The quantity of heat produced from the combustion of 1 Kg of methanol
D ) The quantity of heat produced from the combustion of methanol is no affected by the available amount of oxygen

11)
A) An exothermic process for a quantity of heat equals 571.8 KJ
B) An exothermic process for a quantity of heat equals 285.9 KJ
C) An endothermic process for a quantity of heat equals 571.8 KJ
D) An endothermic process for a quantity of heat equals 285.9 KJ
{"name":"Chemistry 1st term", "url":"https://www.quiz-maker.com/QPREVIEW","txt":"Welcome to the Chemistry 1st Term Quiz! This engaging quiz consists of 17 questions designed to challenge your understanding of fundamental chemistry concepts. Test your knowledge and see how well you can answer questions related to chemical systems, enthalpy, and more!Quiz Features:17 thought-provoking questionsMultiple choice answers for each questionInstant feedback upon completion","img":"https:/images/course1.png"}
More Quizzes
Chemical Reaction Quiz
1166
Biological and Chemical Fundamentals Quiz
1167
GENERAL CHEMISTRY 2 ONLINE TEST
GOOD DAY GRADE 12 STEM STUDENTS!
Here is your 15- item test on the last two lessons we had in Chemistry. You can only answer this test ONCE. so BE CAREFUL ON PUTTING YOUR FINAL ANSWER. After accomplishing the test, please take a screen shot of your final quiz score
Good luck and Thank you! - Ms. Corpin
15822
Types of Materials
5240
Changes of Matter
4219
Miss Tye's Science Quiz 2
1058
Bio
9413
SUBSTANCES, THEIR PROPERTIES AND CLASSIFICATIONS
271441
CHEMISTRY SS1
11625
General Knowledge Challenge
1059
Sedimentation & Decantation.
4233
LUKONYI BOYS PRIMARY SCHOOL STD 8 SCIENCE ASSIGNMENT FOR WEEK 1
2512127





