AP Chem Midterm

AP Chemistry Midterm Quiz
Test your knowledge and understanding of essential concepts in AP Chemistry with this comprehensive midterm quiz. Designed for students preparing for their exams, this quiz features a variety of questions that cover critical topics in chemistry.
Each question is crafted to challenge your understanding and application of chemical principles, helping you to identify areas of strength and those that may require further study. Be ready to engage with:
- Multiple-choice questions
- Topics from elements to molecular geometry
- A total of 40 thought-provoking questions
The atoms of an element, X, has the electron configuration below. What compound would element X most likely form with magnesium (Mg)?
X: 1s22s22p63s23p3
Mg X
Mg_2 X
Mg X_2
Mg_3 X_2
In which of the following compounds is the mass ratio of chromium to oxygen closest to 1.6 to 1.0?
Cr O_3
Cr O_2
Cr_2 O
Cr_2 O_3
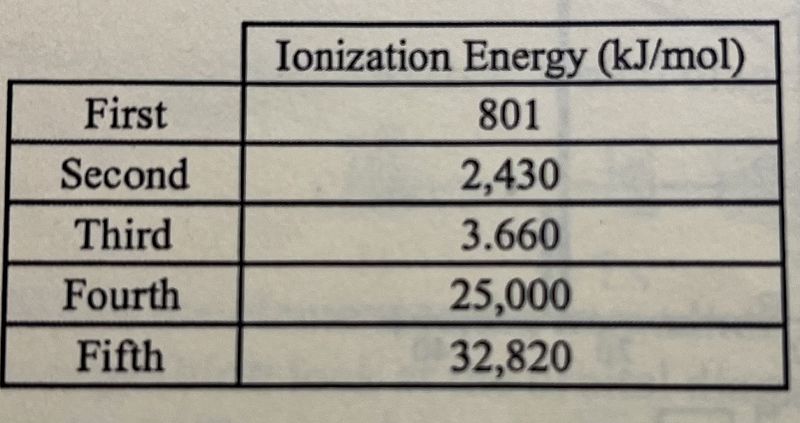
The first five ionization energies of a second-period element are listed in the table above. Which of the following correctly identifies the element and best explains the data in the table?
B, because it has five core electrons
B, because it has three valence electrons
N, because it has five valence electrons
N, because it has three electrons in the p-sublevel
Styrene, a compound used to make Styrofoam cups (MM = 104 g/mol) contains 92.3% C and 7.75% H. What is styrene's molecular formula?
C H
C_8 H_8
C_4 H_8
C H_2
A measured mass of an unreactive metal was dropped into a small graduated cylindwer half filled with water. The following measurements were made.
Mass of metal = 19.611 grams
Volume of water before addition of metal = 12.4 mL
Volume of water after addition of metal = 14.9 mL
The density of the metal should be reported as:
7.8444 grams per mL
7.84 grams per mL
7.8 grams per mL
8 grams per mL
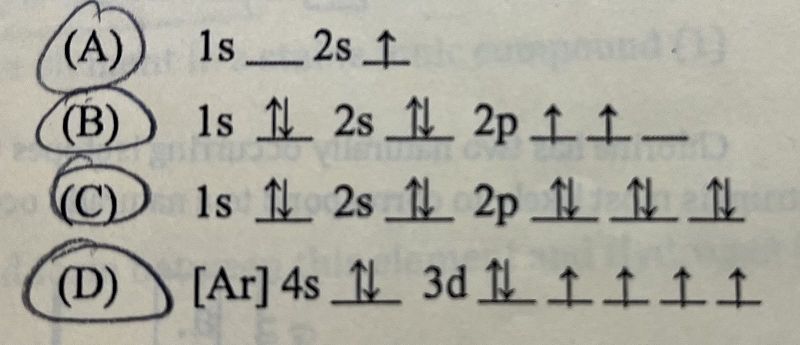
Which electron configuration represents an atom in an excited state?
A
B
C
D
Copper (II) sulfate pentahydrate, CuSO4•5H2O (molar mass: 250 g/mol) can be dehydrated by repeated heating in a crucible. Which value is closest to the percentage mass of water lost from the total mass of salt in the crucible when the crucible undergoes reptitive heatings until a constant mass is reached?
13%
25%
26%
36%
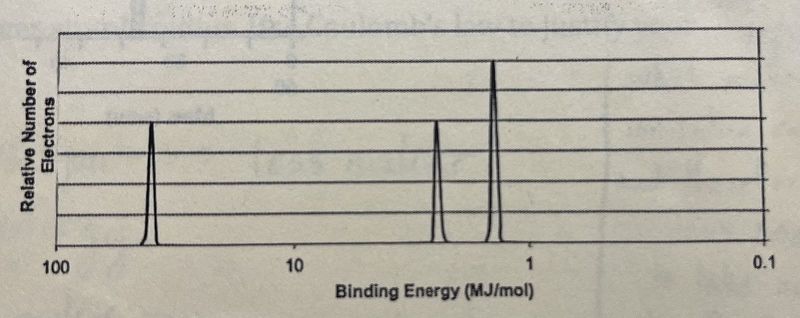
The element in photoelectron spectroscopy graph, above, is most likely
Li
Be
N
Ne
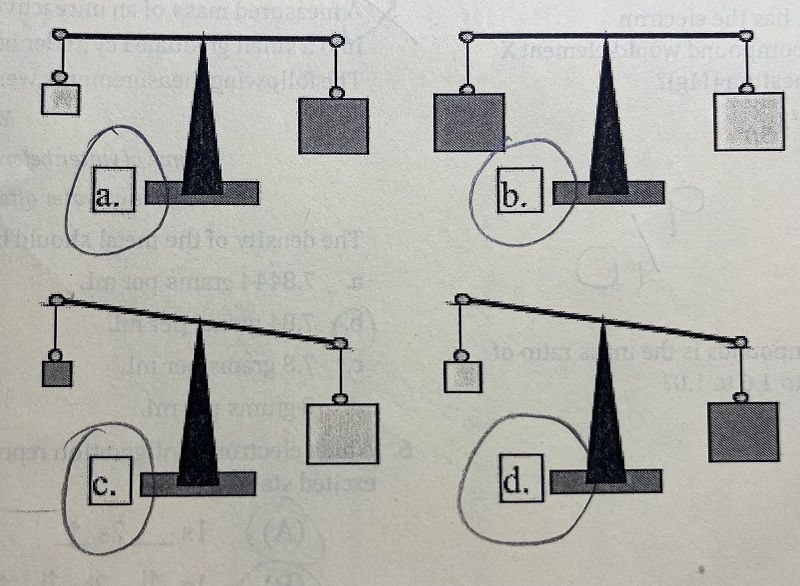
In which balance and block arrangement above, can you be sure the lighter colored block is more dense?
A
B
C
D

Chlorine has two naturally occuring isotopes with masses of 34.969 amu and 36.966 amu. Which mass spectrum is most likely to correspond to a naturally occurring sample of chlorine?
A
B
C
D
Which of the following correctly lists the compounds in order of increasing lattice energy?
KCl < CsI < MgO < CaO
CsI < KCl < CaO < MgO
CaO < MgO < CsI < KCl
KCl < MgO < CaO < CsI
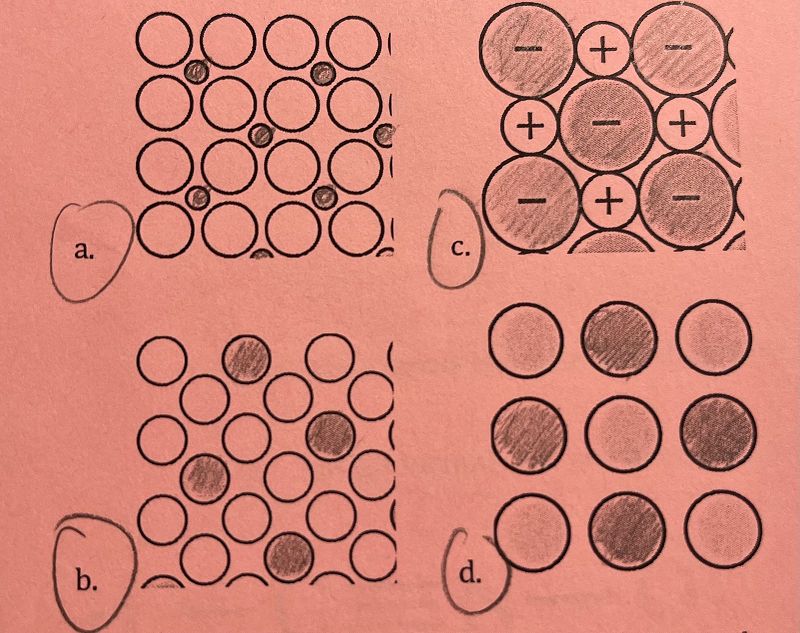
Copper atoms and zinc atoms have the same atomic radius, 135 picometers. Based on this information, which of the following diagrams best represents an alloy containing only copper and zinc atoms?
A
B
C
D

Which of the following is the most favorable Lewis dot structure for SOCl2, considering formal charge?
A
B
C
D
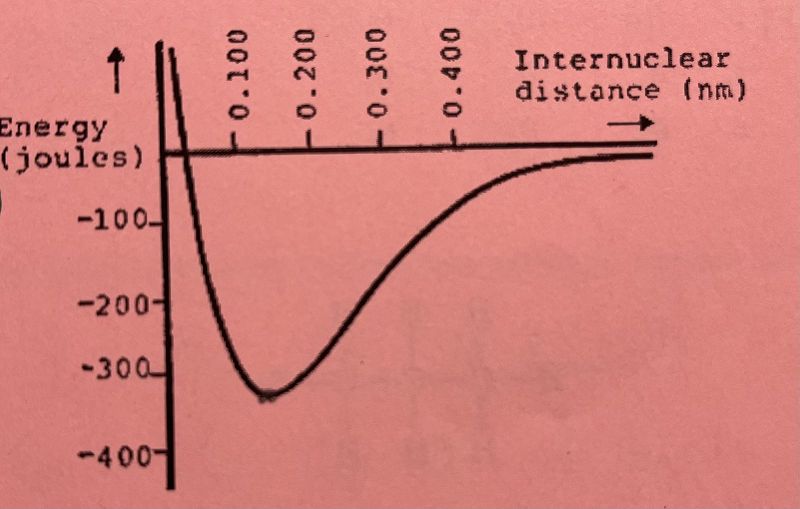
What is the approximate bond length between the two atoms in the energy diagram?
0.020 nm
0.140 nm
0.400 nm
0.330 nm
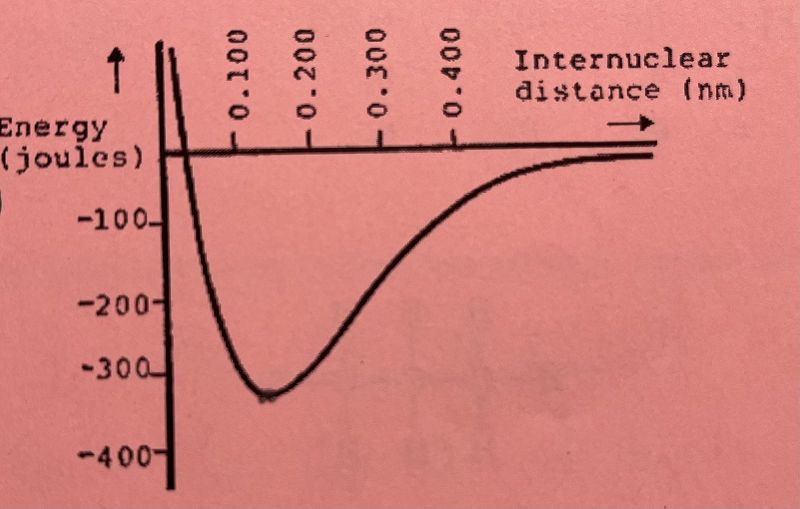
In the energy diagram above (between two atoms), what is the bond energy (bond strength) of the bond?
1100 J
10 J
330 J
422 J
What is the electron geometry of H2O?
Linear
Octahedral
Bent
Tetrahedral
What is the hybridization of one of the carbon atoms in C2H4?
Sp
Sp^2
Sp^3
Sp^3 d^2
What is the molecular geometry of BrI3?
Square planar
T-Shaped
Trigonal Bipyramidal
See-saw
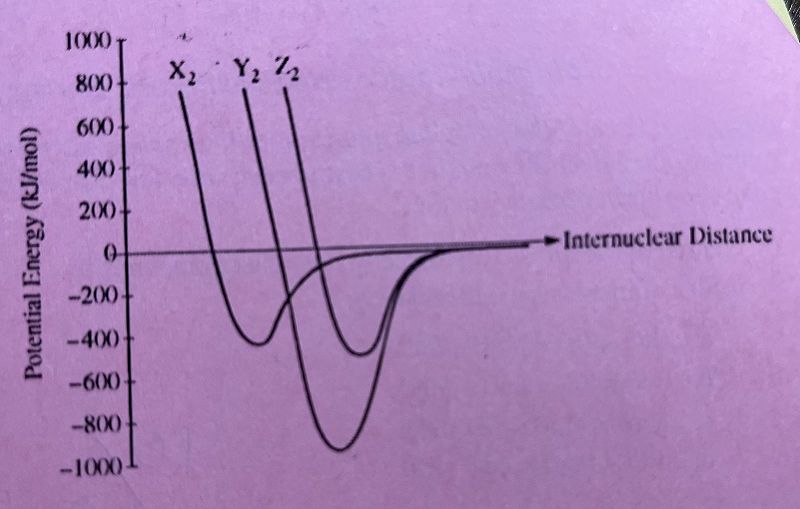
The potential energy as a function of internuclear distance for three diatomic molecules, X2, Y2, Z2, is shown in the graph. Based on the data in the graph, which of the following correctly identifies the diatomic molecules X2, Y2, Z2 in order?
H2, N2, O2
H2, O2, N2
N2, O2, H2
O2, H2, N2
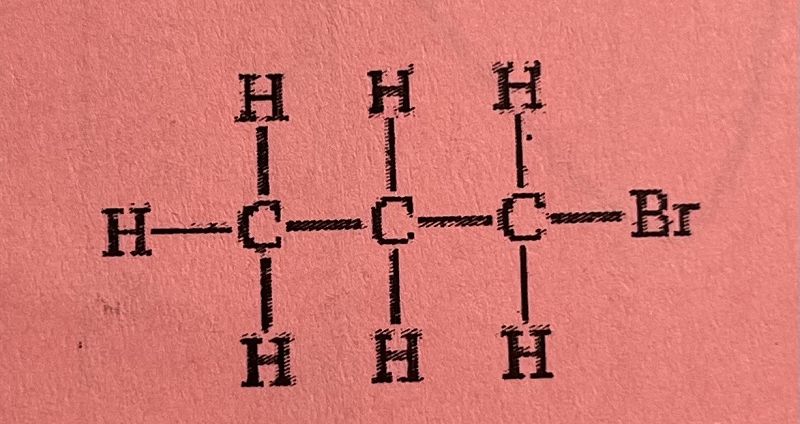
Which of the following Lewis structures of C3H7Br represents an isomer of the compound that has the Lewis structure shown above?
0%
0
0%
0
0%
0
0%
0
D
Which of the following has the bonds arranged in order of decreasing polarity?
Sb-I > Sb-T > Sb-Cl
O-N > O-S > O>Te
H-I > H-Br > H-F
H-F > N-F > F-F
A 50.0 mg sample of calcium bromide, CaBr2, (molar mass 200. g) is dissolved in enough water to produce 200. mL of solution. What is the molarity of calcium bromide in a 50. mL sample of this solution?
0.00250 M
0.00125 M
0.00500 M
0.00200 M
Which of the following molecules has a dipole moment of zero?
S O_2
N O
N H_3
C F_4
The boiling points of the elements helium, neon, argon, krypton, and xenon increase in that order. Which of the following statements best account for this increase?
The hydrogen bonding increases
The dipole-dipole forces increase
The molar mass increases
The (London) dispersion forces increase
Which gas listed below has anaverage atomic or molecular speed closest to that of N2 molecules at 0°C and 1 atm?
CO
NO
O_2
Ne
Equal masses of three different ideal gases, X, Y, Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct?
It is equal to 1/3 of the total pressure
It depends on the average distance traveled between molecular collisions
It can be calculated with knowledge only of the volume of the container
It depends on the molecular masses of X, Y, Z
Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 gram of hydrogen and the other holds 8.0 grams of oxygen. Which of the following statements regarding these gas samples is FALSE?
The density of the hydrogen sample is less than that of the oxygen sample
The number of molecules in the ydrogen container is the same as the number of molecules in the oxygen container
The average speed of the hydrogen molecules is the same as the average speed of the oxygen molecules
The volume of the hydrogen container is the same as the volume of the oxygen container
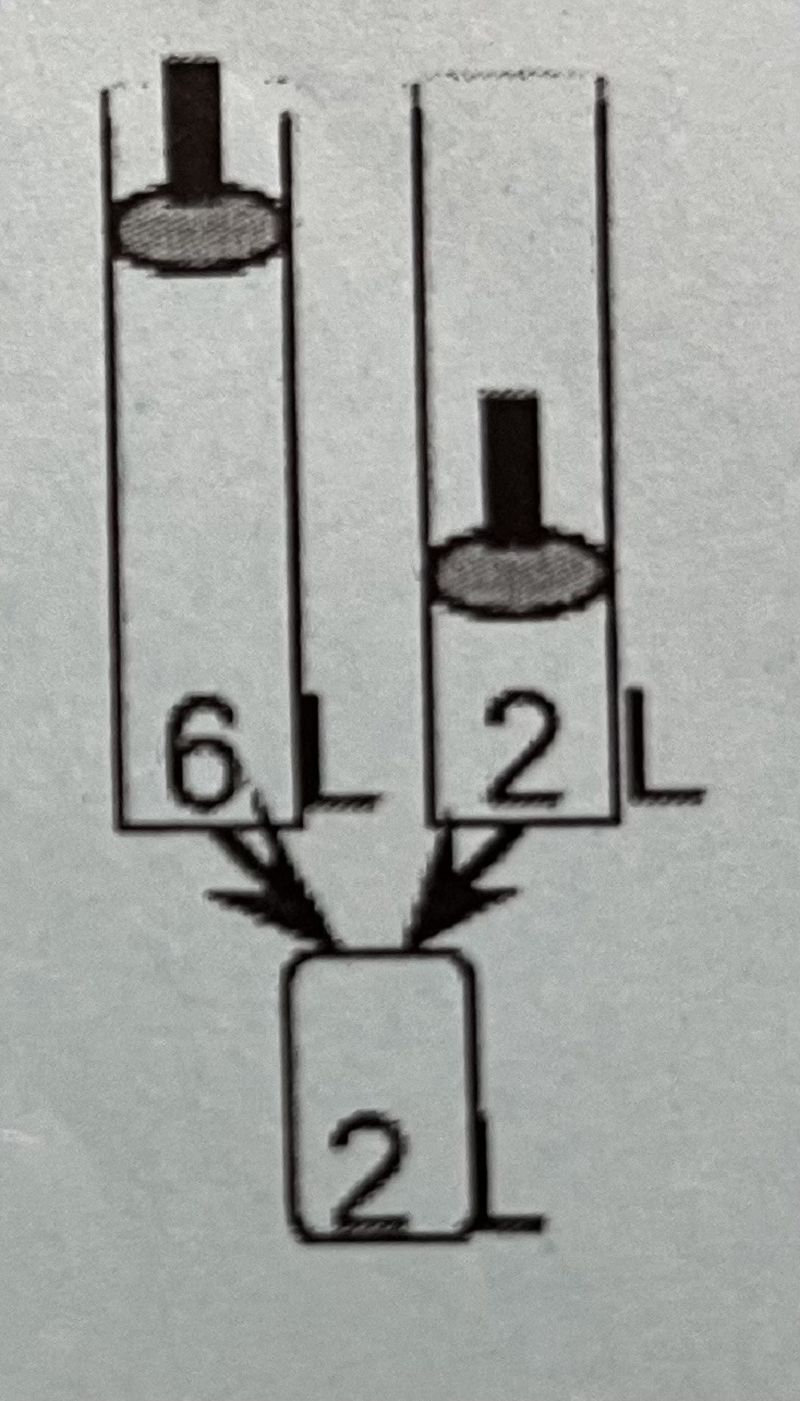
A 2L sample of N2 (g) and a 6L sample of Ar (g), each originally at 1 atm and 0°C, are combined in a 2L tank. If the temperature is held constant, what is the total pressure in the 2L tank?
8 atm
4 atm
2 atm
1.3 atm
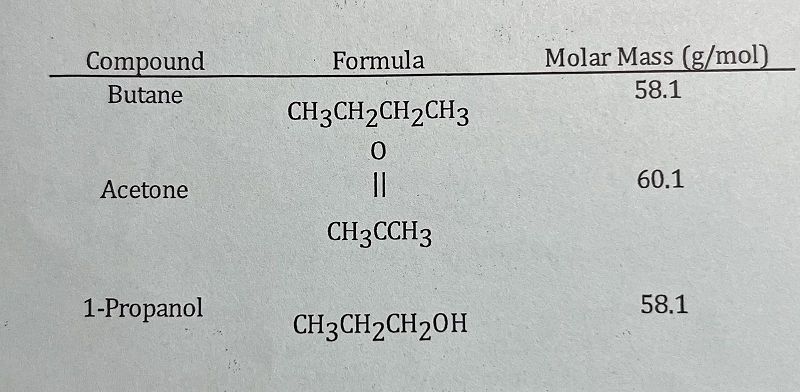
The table above shows the structural formulas and molar masses for three compounds. Which of the following is a list of the compounds in order of increasing boiling points?
Acetone = butane < 1-propanol
1-propanol < acetone < butane
Butane < acetone < 1-propanol
Butane < 1-propanol < acetone
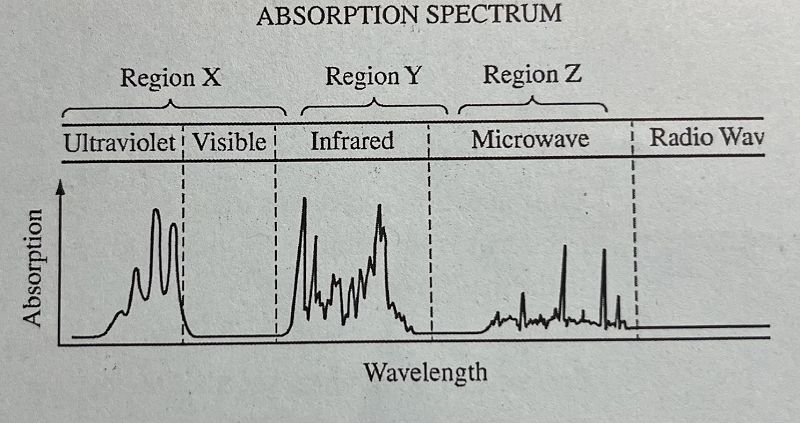
The diagram above represents the absorption spectrum for a pure molecular substance. Which of the following correctly indicates the type of transition observed for the substance in each of the regions of the absorption spectrum? Listed in order of Region X, Region Y, Region Z
Molecular vibration, molecular rotation, electron transition
Electron transition, molecular rotation, molecular vibration
Molecular rotation, molecular vibration, electron transition
Electron transition, molecular vibration, molecular rotation
Which of the following is LEAST soluble in water?
KMnO_4
(NH_4)_2 SO_4
Na_3 PO_4
BaCO_3
When 100 mL of 1.0 M AlCl3 is mixed with 100mL of 1.0 M Na2CrO4, a yellow precipitate formsand [CrO4-2] becomes negligibly small. Which of the following is a correct listing of the ions remaining in solution in order of increasing concentration?
[Al] < [Na] < [Cl]
[Cl] < [Na] < [Al]
[Al] < [Cl] < [Na]
[Al] < [Cl] = [Na]
An aqueous solution contains 0.6 mol KCl and 1.20 mol NH4Cl. What is the minimum number of moles of Pb(NO3)2 that must be added to precipitate all of the chloride ions?
0.30 mol
0.45 mol
0.60 mol
0.90 mol
The unbalanced products of the combustion of sucrose (C12H22O11) are...
CO2 and H2O
SO2 and OH
SO and H2O
NO2 and H2O
Predict the balanced products of the following reactinon: Chlorine gas is bubbled through a solution of potassium bromide
K + Cl_2 Br_2
KCl + Br
2KCl + Br_2
KBr + Cl
A strong acid is added to a strong base. What is the net ionic equation of the reaction?
H (aq) + OH (aq) →H2O (l)
2H (aq) + 2OH (aq) → 2H_2 (g) + O_2 (g)
Depends upon the identity of each reactant
Depends upon the amount of each reactant
How many electrons appear in the following half-reaction when it is balanced?
12 H+ + 2IO3- → I2 + 6H2O
5e on the right
5e on the left
10e on the right
10e on the left
In which of the following species does sulfur have the same oxidation number as it does in H2SO4?
S_2 ^(2-)
S_2 O_3 ^(2-)
SO_2 Cl_2
H_2 SO_3
__H+ + __NO3- + __Mg → __Mg2+ + __NH4+ + __H2O
When the equation above is balanced with lowest whole number coefficients, the coefficient for H+ is:
10
8
6
4
2 H2O + 4 MnO4- + 3 ClO2- → 4MnO2 + 3 ClO4- + 4OH-
MnO4 ^(1-)
OH ^(1-)
H2O
ClO_2 ^(1-)
{"name":"AP Chem Midterm", "url":"https://www.quiz-maker.com/QPREVIEW","txt":"Test your knowledge and understanding of essential concepts in AP Chemistry with this comprehensive midterm quiz. Designed for students preparing for their exams, this quiz features a variety of questions that cover critical topics in chemistry.Each question is crafted to challenge your understanding and application of chemical principles, helping you to identify areas of strength and those that may require further study. Be ready to engage with:Multiple-choice questionsTopics from elements to molecular geometryA total of 40 thought-provoking questions","img":"https:/images/course1.png"}
More Quizzes
Types of Bonding Quiz
840
Md-term exam | Ahmad Albedaw & Obadah Alzoubi |
2010605
Academic Year 2020-2021 IP Orientation Quiz
2010606
What Medieval Item Are You?
840
Greek Architecture - Columns, Orders & Temples
201019351
Will We Get Back Together - Free Instant Results
201018686
Which Pevensie Sibling Are You? Narnia Character
201016547
Subway Sandwich - How Well Do You Know the Menu?
201017016
Food Handlers Card Arizona - Free Practice Test
201019894
Body Regions - Test Your Anatomy Knowledge (Free)
201019264
Anatomy Landmarks - Free Online Practice
201018928
Which Disciple Are You? Free Bible Personality
201017460