PHYSICS-Heat & Thermodynamics (SMART INSTITUTE)


01-The molecules of an ideal gas at thermodynamics (absolute) temperature T have a root-mean-square speed Crms. The gas is heated to temperature 2T. What is the new root-mean-square speed of the molecules?
�2Crms
2 Crms
2√2 Crms
4 Crms
02-The average kinetic energy of hydrogen molecules at 300 K is 'E'. At same temperature K.E of oxygen molecules will be:
E/16
E
E/4
4E
03-Which statement about the first law of thermodynamics is correct?
The heating of a system equal to the increase of its internal energy plus the work done on the system
The increase in the internal energy of the system equal the heating of the system plus the work done by the system
The increase in the internal energy of a system equal the heating of the system minus the work done by the system
The work done on a system equals the increase of its thermal energy plus the heating of the system
04-The temperature of an ideal gas is increased from 120 K to 480 K. If at 120 K rms speed is 'v' then at 480 K, it will:
2v
4v
V/2
V/4
05-The kinetic energy of molecular motion appears as:
P.E
Temperature
Heat
With P.E or Heat
06-If amount of heat given to a system be 35J and then amount of work done by the system be +15J, then change in the internal energy of the system is:
-50 J
-20 J
+20 J
50 J
07-During an adiabatic expansion, the increase in volume is associated with:
Decrease in pressure and decrease in temperature
Increase in pressure and increase in temperature
Increase in pressure and decrease in temperature
Decrease in pressure and increase in temperature
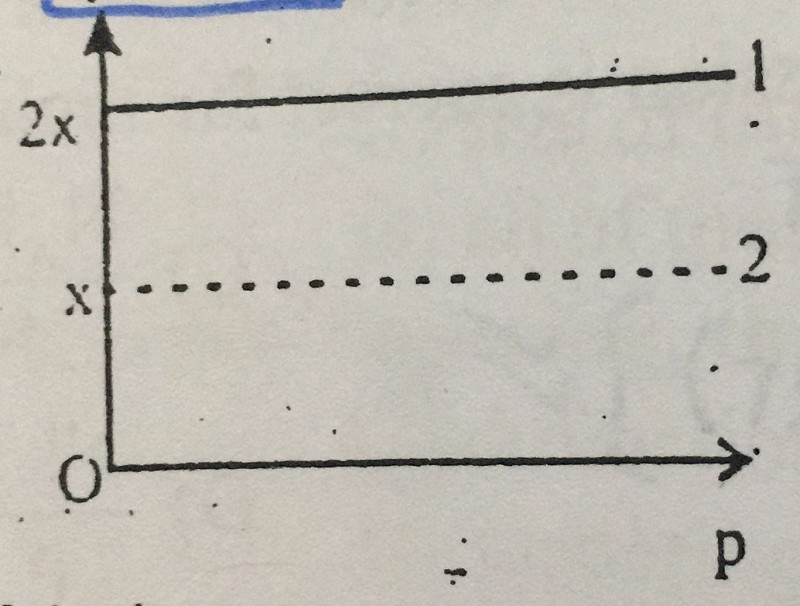
08-The relationship between volume V and pressure P for a sample of gas at a given temperature is shown by the isotherm marked 1 in figure given below. Isotherm 2 would be followed by the gas if:
The mass of the gas was doubled
The thermodynamics temperature of the gas was doubled
The thermodynamics temperature of the gas was halved
The mass of the gas was doubled and its thermodynamics temperature was halved
09- 1/273.16 of thermodynamic temperature of the triple point of water is equal to:
1 'C
1K
0'C
0K
10-An ideal gas is compressed at constant temperature. Which of the table is correct?
Work is done by gas, heat energy goes out of gas
Work is done by gas, heat energy goes into gas
Work is done on gas, heat energy goes into gas
Work is done on gas, heat energy goes out of gas
11-The temperature at which the kinetic energy of a gas molecule is double. Its value at 27'C is
54 'C
327'C
300 K
108'C
12-What does V represent in the equation PV'/2 = RT
Volume of n moles of gas
Mass of 2 gm of gas
Mass of 4g of gas
Volume of 2 moles of gas
13-As Cp-Cv=R shows that Cp>Cv. What is also true?
А��Tp>𐤃Tv
А��Up>𐤃Uv
Both of above
А��Up=𐤃Uv
14-If the temperature of a patient is 40'C, his temperature on the Fahrenheit scale will be
72'
96'
100'
104'
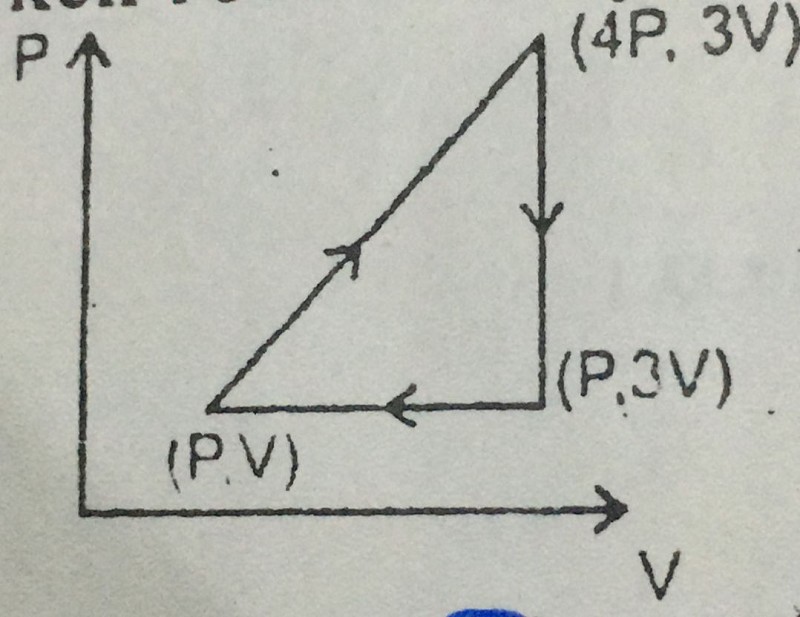
15-An ideal monoatomic gas has taken round the cycle, work done during the cycle is:
0
6 PV
3 PV
9 PV
16-Boyle's law holds for ideal gases in
Isochoric processes
Isothermal processes
Isobaric processes
Adiabatic processes
17-The scales of temperature are based on two fixed points, which are:
The temperatures of water at 0'C and 100'C
The temperatures of ice cold and boiling water
The temperature of melting ice and boiling water at atmospheric pressure
The temperatures of frozen and boiling mercury
18-Two gases A and B having the same temperature T, same pressure P and same volume V are mixed. If the mixture is at the same temperature T and occupies a volume V/2 , the pressure of the mixture is:
2P
P/2
P
4P
19-During the adiabatic expansion of 2 moles of a gas, the internal energy of the gas is found to decrease by 2 J, the work done during the process on the gas will be equal to:
-2 J
1 J
2 J
-1 J
20-A polyatomic gas (γ=4/3) is compressed to 1/8 of its volume adiabatically. If its initial pressure is Po, its new pressure will be:
16Po
8Po
6Po
2Po
21-The absolute temperature for the ideal gas is:
Directly proportional to the rotational K.E of the gas molecules
Directly proportional to the vibrational K.E of the gas molecules
Directly proportional to the average transltional K.E of the gas molecules
Sum of K.E of all molecules
22-Which one is true for internal energy?
It is sum of all forms of molecular energies of a system
It is proportional to translational K.E of the molecules
It is a state function of a system
All are correct
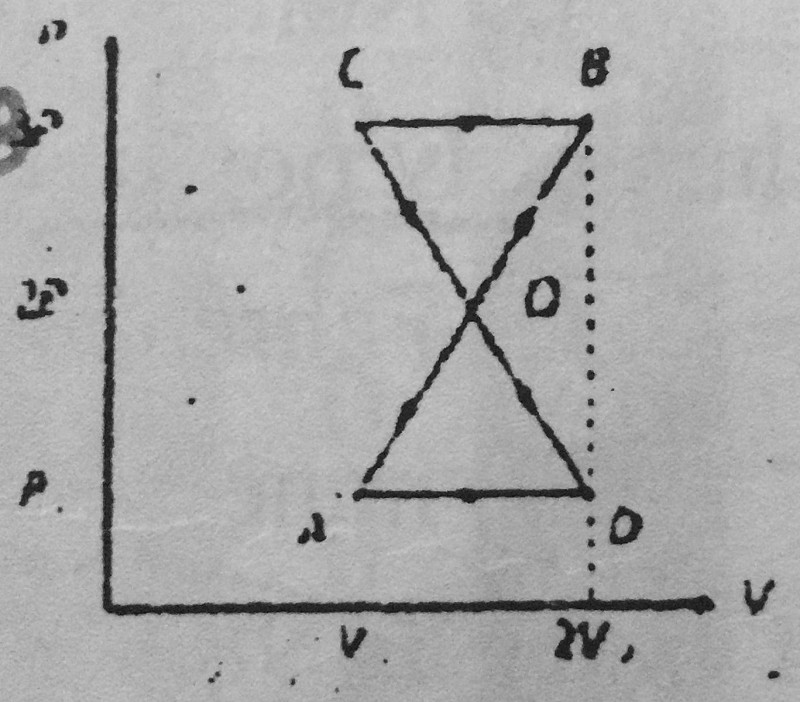
23-A thermodynamic system undergoes cyclic process ABCDA as shown in figure. The work done by the system is:
2PoVo
PoVo
PoVo/2
Zero
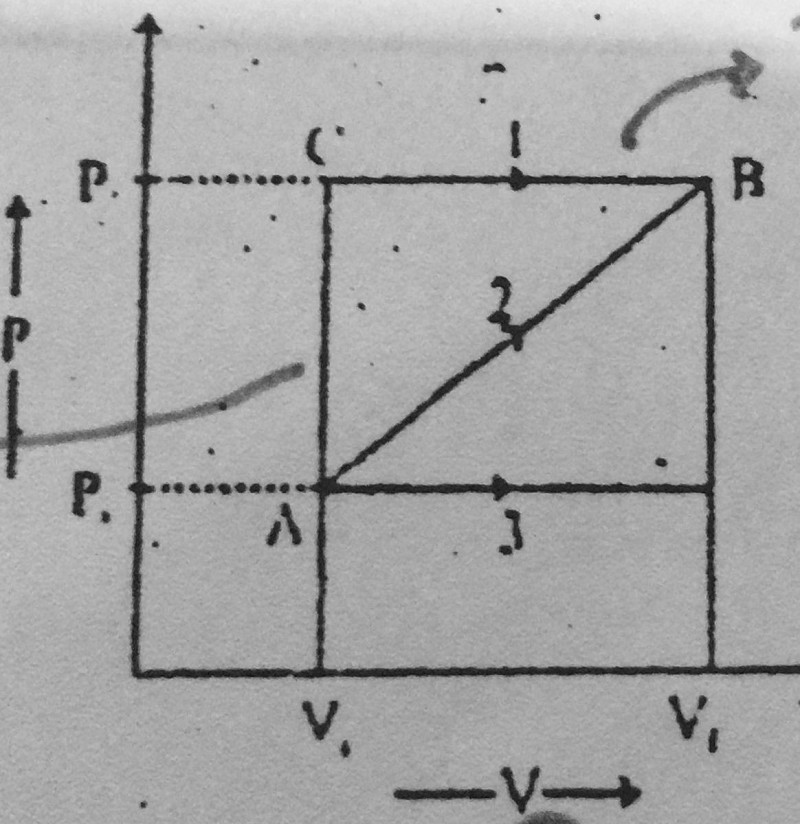
24-A system is taken from state A to B through three different paths 1,2,3. The work done is maximum in:
Process 3
Process 2
Process 1
Equal in all processes
25-A gas is compressed from a volume of 2m^3 to a volume of 1 m^3 at a constant pressure of 100 N/m^2. Then it is heated at constant volume by supporting 150 J of energy. As a result, the internal energy of the gas
Decreases by 250 J
Increases by 250 J
Increases by 50 J
DECREASES BY 50 J
26-One mole of a perfect gas in a cylinder fitted with a piston has a pressure P, volume V and temperature T. If the temperature is increased by 1 K keeping pressure constant, the increase in volume is:
2V/273
V/273
V/91
V
27-During an adiabatic expansion of 2 moles of gas, the internal energy was found to decrease by -100J. The work done by the gas during the process will be equal to:
100 J
-100 J
ZERO
200 J
28-A gas is compressed at a constant pressure of 50 N/m2 from a volume of 10 m3 to a volume of 4 m3. Energy of 100 J then added to the gas by heating. Its internal energy is:
Increased by 400 J
Increased by 100 J
Increased by 100 J
Decreased by 200 J
29-Work done by air when it expands from 50 L to 150 L at a constant pressure of 2 atm is:
2 x 10^4 J
2 x 100 J
2 x 10^5 X 100 J
2 x 10^-5 X 100 J
30-Temperature remaining constant, the pressure of gas is decreased by 20. The percentage change in volume:
Increases by 20
Increases by 25
Decreases by 20
Decreases by 25
31-At absolute temperature, the K.E of the molecules
Become minimum
Become zero
Become maximum
None of these
32-Temperature is a property, which determines
How much heat a body contains
Whether a body will feel hot or cold to touch
In which direction heat will flow between two systems
How much total absolute energy a body has
33-WOF thermometers has only positive degrees of temperature?
Celsius
Kelvin
Fahrenheit
None of these
34-The temperature of a normal human body is 98.6'F. This temperature on centigrade scale is:
0'C
73'C
37'C
37.6'C
35-The direction of flow of heat between two bodies depends upon:
Thermal conductivity
Internal energies
Specific heat
Temperature difference
36-The statement "it is impossible for a self-acting machine, to transfer heat from a lower temperature to higher temperature" obeys
1st law of thermodynamics
Law of conservation of momentum
2nd law of thermodynamics
Law of conservation of energy
37-The internal energy of a body is maximum when its temperature is:
0K
-273K
273 K
-273'C
38-Internal energy depends upon
Final state
Initial state
Both of above
None of these
39-By rubbing the objects together, their internal energy
Increases
Remains constant
Decreases
Becomes zero
40-Work done by the system on its environment is taken is
Positive
Neutral
Negative
None of these
41-In an ideal gas, the molecules possess
Only K.E
K.E and P.E both
Only P.E
Only gravitational energy
42-If an ideal gas is isothermally expanded, its internal energy will:
Increase
Decrease
Remain same
Decrease or increase depending on the nature of the gas
43-Cp-Cv and Cv/Cp are respectively equal to
[1/R, γ^-1]
(R, g)
[1/R, γ]
(R, γ^-1)
44-First law of thermodynamics can be written as CvΔT=ΔQ-PΔV. In a change for which Boyle's law is observed, WOF would necessarily zero?
ΔQ
ΔT
ΔV
Cv
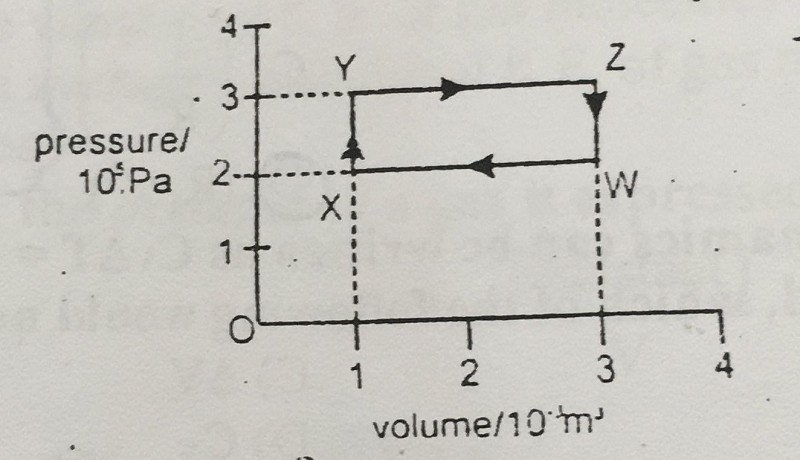
45-A gas undergoes the cycle of pressure and volume changes W→X→Y→Z→W as shown in diagram. What is the net work done by the gas?
-600 J
-200 J
0 J
200 J
46-Cp-Cv value, find if 3 moles of gas is given.
3R
5R
2R
7R
47-Gas laws are applicable to
Gases as well vapours
Gases and steam
Gases alone and not to vapours
Gases and vapours under certain conditions
48-Thermodynamics does not consider
Initial and final state
Bulk of matter
Time factor
Energy change
49-A system can neither exchange matter nor energy with the surroundings is called
Open system
Closed system
Isolated system
Ideal system
50-Absolute zero pressure will be
At sea level
At center of earth
When molecule moment of system is zero
None of these
{"name":"PHYSICS-Heat & Thermodynamics (SMART INSTITUTE)", "url":"https://www.quiz-maker.com/QPREVIEW","txt":"01-The molecules of an ideal gas at thermodynamics (absolute) temperature T have a root-mean-square speed Crms. The gas is heated to temperature 2T. What is the new root-mean-square speed of the molecules?, 02-The average kinetic energy of hydrogen molecules at 300 K is 'E'. At same temperature K.E of oxygen molecules will be:, 03-Which statement about the first law of thermodynamics is correct?","img":"https://www.quiz-maker.com/3012/CDN/79-3702469/whatsapp-image-2022-07-16-at-3-44-56-pm.jpeg?sz=1200"}
More Quizzes
Inspector Calls
520
Kim and Mr. Green's Adventure
1478
WHICH GUARD-RAIL BRAT ARE YOU?
11612
̤�국 개발사가 개발에 참여한 모바일게임일까요?
630
Por vs Para Practice - Master Spanish Prepositions
201021831
Rat Dissection - Identify Organs & Functions
201027777
Montana Trivia - Test Your Big Sky State Knowledge
201029154
Google Government Exam Practice (Free)
201019510
Can You Ace These Super Bowl Trivia Game Questions?
201037368
Am I a Bad Daughter - Free Self‑Assessment
201022418
True Lies Trivia - How Well Do You Remember?
201025240
Criminal Psychology Test - Forensic Psychology Research
201024452