Placement Test TBAT Chemistry 2024
Question 1
Question 2
Question 3
Which of the following has the lowest oxidation number of Cl?
Question 4
Several chemical reactions have occurred apparently. Which of the following reactions give the product as solid?
- The reaction of lithium and excess water at room temperature.
- The reaction of 100 mL of calcium chloride and 100 mL of potassium nitrate solution in a beaker.
- The decomposition reaction of heating limestone.
- The reaction of 0.50 M potassium bromide solution and 0.50 M lead(II) nitrate solution.
Question 5
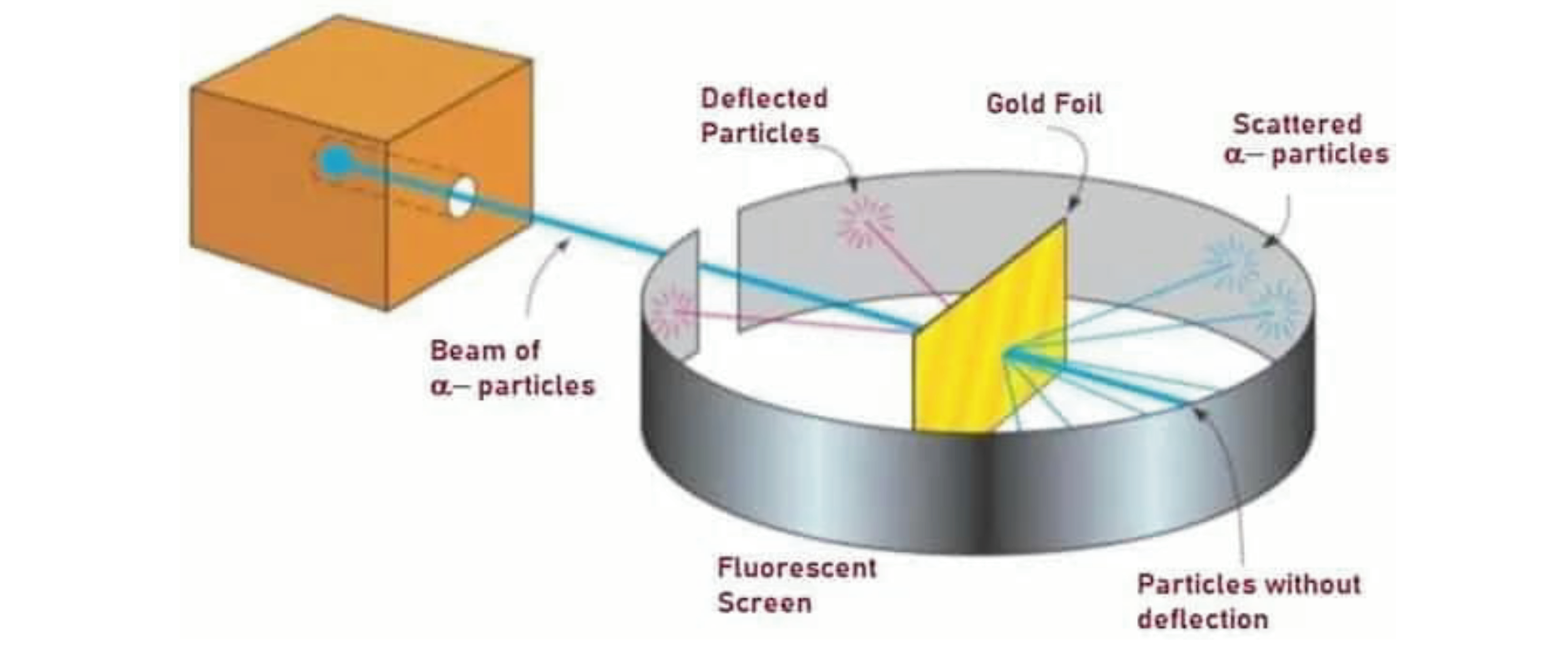
Question 6
Question 7
Question 8
Question 9
Question 10
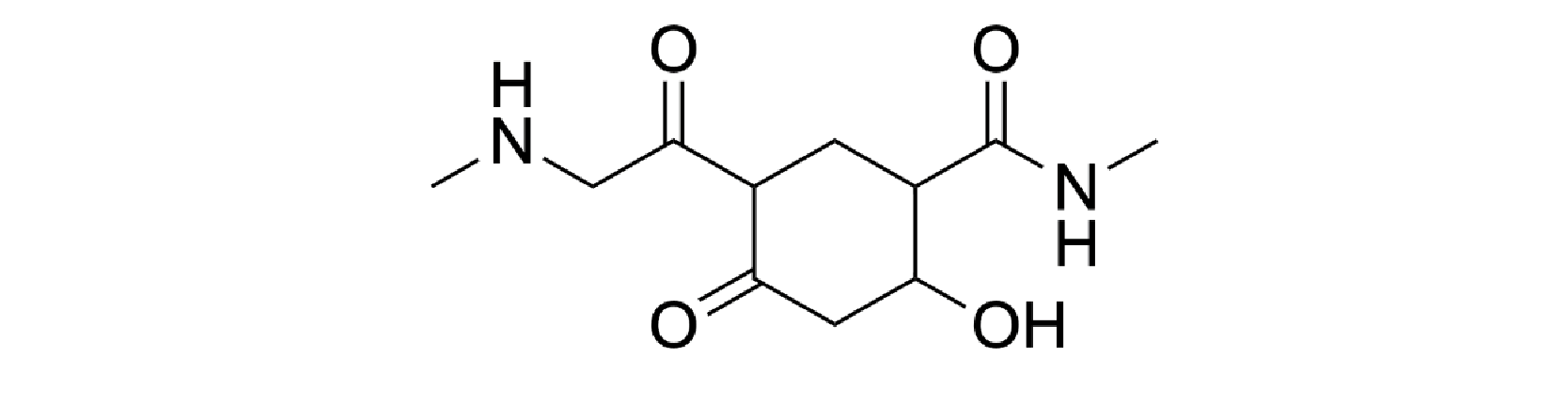
Question 11
A 12-mole mixture of oxygen and carbon dioxide has a total mass of 480 g. How many moles of carbon dioxide are present in the mixture? (Relative atomic mass of C = 12, O = 16)
Question 12
Question 13
Question 14
Question 15
Question 16
(Relative mass of H = 1, C = 12, O = 16, unknown hydrocarbon = 42)
Question 17
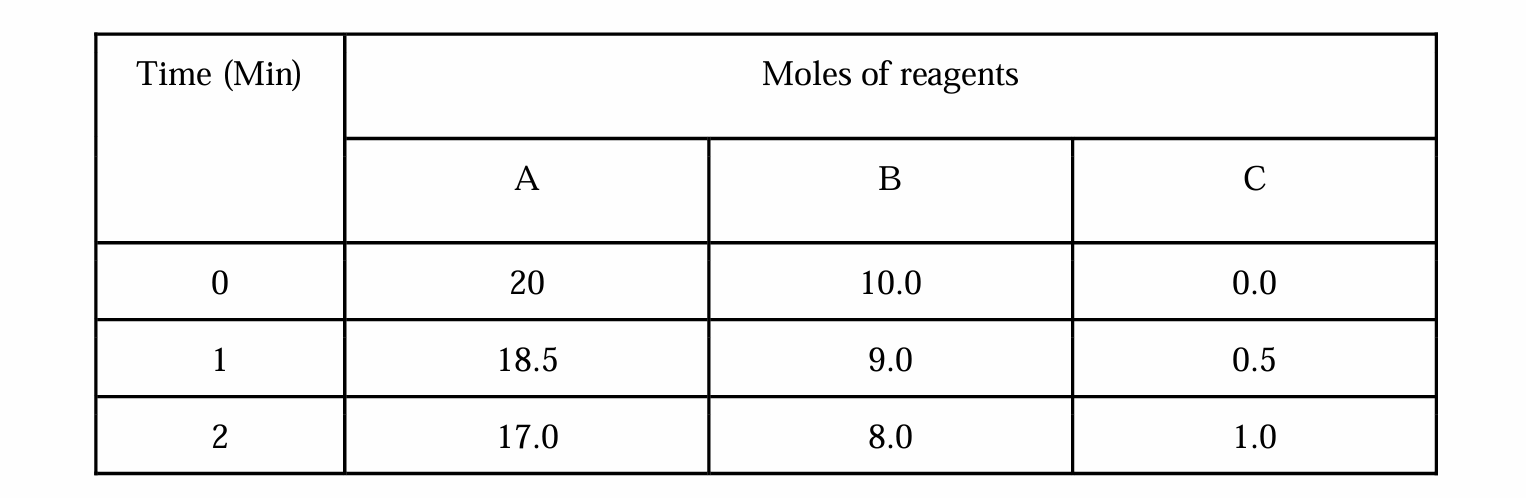
What is the balanced equation, and how long does it take to complete the reaction?
Question 18
2ICl (g) + H2 (g) → I2 (g) + 2HCl (g)
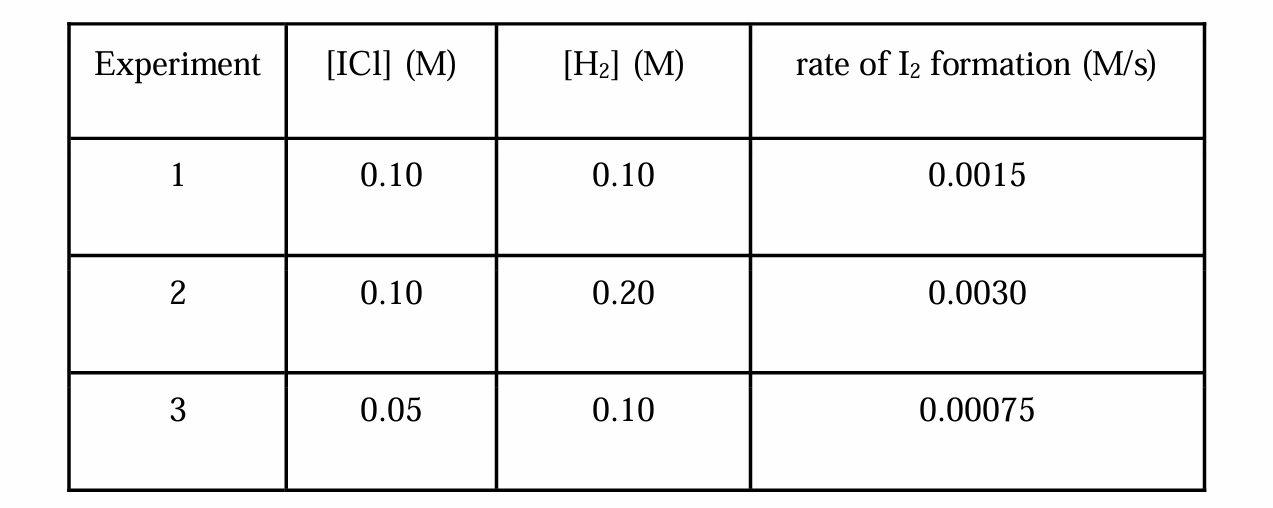
Which one is the reaction rate (R) when the initial concentration of ICl and H2 gas is equal to 0.20 M?
Question 19
Question 20
1.44 x 10-3 mol dm-3. At equilibrium, 27.0 g of SO2Cl2 was found in the container that has the volume of 2.00 dm3. What is the initial amount of SO2Cl2? (Relative atomic mass of O = 16, S = 32, Cl = 35.5)