Placement test IGCSE Chemistry V01
|
solid |
W ⇋ Z |
liquid |
X ⇋ Y |
gas |
Which process, W, X, Y, or Z, is occurring in the following four situations?
- The volume of liquid water in an open beaker reduces when placed under the sun.
- Water condenses on a cold bottle of water.
- Water in a lake freezes during winter.
- Butter melts on a warm day.
|
|
1. |
2. |
3. |
4. |
|
A |
W |
X |
Y |
Z |
|
B |
W |
Y |
X |
Z |
|
C |
X |
Y |
Z |
W |
|
D |
X |
Z |
Y |
W |
|
E |
I don’t have any ideas. |
|||
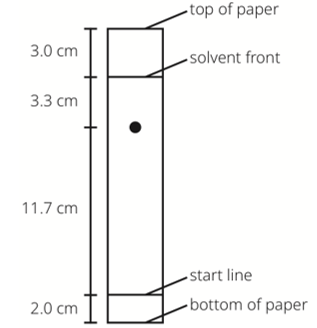
What is the Rf value for this food dye?
|
|
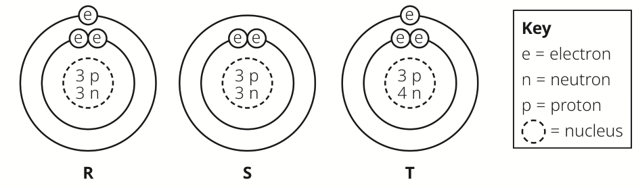
atoms |
isotopes |
|
A |
R |
S and T |
|
B |
R |
R and S |
|
C |
R and T |
R and T |
|
D |
R and T |
R, S, and T |
|
E |
I don’t have any ideas. |
|
Which words correctly complete gaps 1, 2, and 3?
|
|
1 |
2 |
3 |
|
A |
SrCl |
different form |
ionic |
|
B |
SrCl |
the same as |
covalent |
|
C |
SrCl2 |
different form |
ionic |
|
D |
SrCl2 |
the same as |
covalent |
|
E |
I don’t have any ideas. |
||
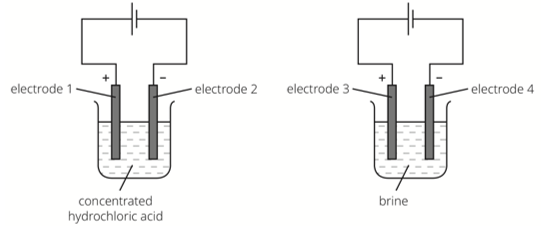
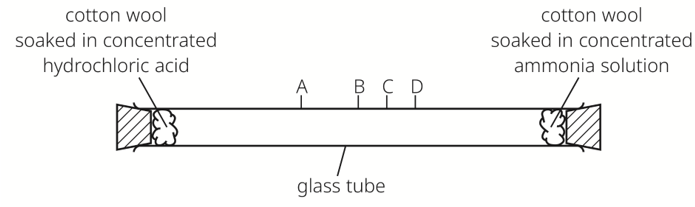
At which electrode(s) is hydrogen produced?
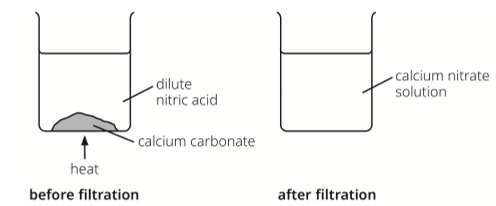
What was the pH of the solution added by the calcium carbonate before and after filtration?
|
|
before filtration |
after filtration |
|
A |
greater than 7 |
7 |
|
B |
less than 7 |
less than 7 |
|
C |
less than 7 |
7 |
|
D |
7 |
greater than 7 |
|
E |
I don’t have any ideas. |
|
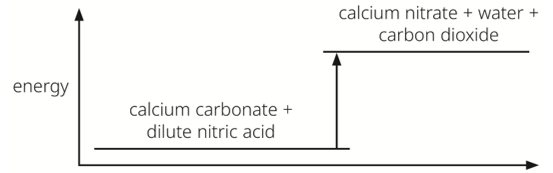
Which row correctly describes the type of reaction and the energy of the reactants and products?
|
|
type of reaction |
energy of the reactants and products |
|
A |
endothermic |
the products have more energy than the reactants |
|
B |
endothermic |
the reactants have more energy than the products |
|
C |
exothermic |
the products have more energy than the reactants |
|
D |
exothermic |
the reactants have more energy than the products |
|
E |
I don’t have any ideas. |
|
The hydrogen gas is collected and its volume measured.
The results are shown on the graph.
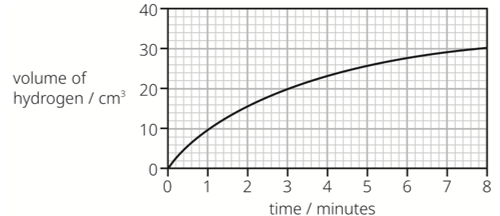
What is the volume of hydrogen produced during the fourth minute?
2NO2(g) ⇋ N2O4(g) ΔH = - 58 kJ/mol
Which statement about an equilibrium mixture of NO2 and N2O4 is correct?
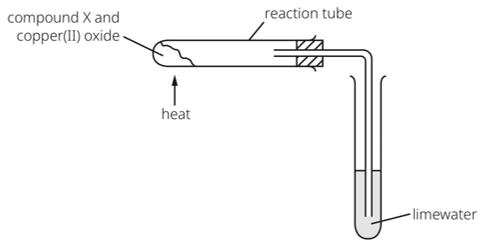
A brown solid copper metal is formed in the reaction tube and the limewater turns cloudy
What is compound X?
|
compound |
formula |
|
W |
FePO4 |
|
X |
NH4NO3 |
|
Y |
K3PO4 |
|
Z |
KBr |
Which mixture of compounds makes a complete fertiliser?
Which substance is poisonous to human bodies?
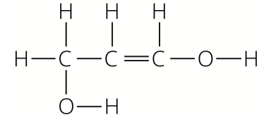
Which fun
|
|
carboxylic acid |
alkene |
alcohol |
|
A |
no |
no |
no |
|
B |
no |
yes |
yes |
|
C |
yes |
no |
yes |
|
D |
yes |
yes |
yes |
|
E |
I don’t have any ideas. |
||
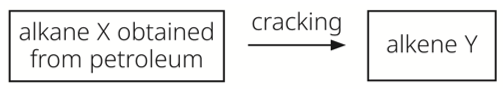
Which row describes the process of cracking?
|
|
boiling point of X |
boiling point of Y |
temperature required |
|
A |
higher |
lower |
low |
|
B |
higher |
lower |
high |
|
C |
lower |
higher |
low |
|
D |
lower |
higher |
high |
|
E |
I don’t have any ideas. |
||
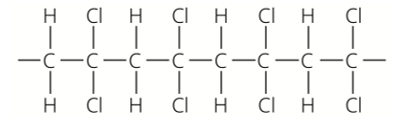
Which alkene monomer is used to make this polymer?
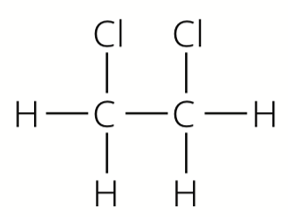
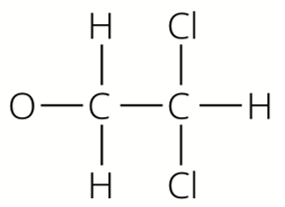
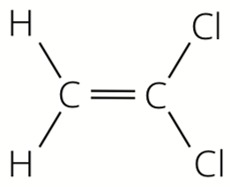
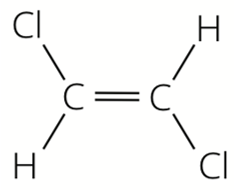
Fat 1 has the formula
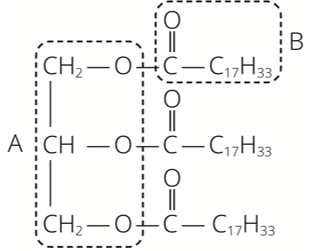
Fat 1 is the product of two types of molecules, A and B. The diagram shows parts of molecules A and B in the structure of Fat 1. Deduce the fun
|
|
fun |
fun |
|
A |
alcohol |
carboxylic acid |
|
B |
carboxylic acid |
alcohol |
|
C |
ester |
alcohol |
|
D |
carboxylic |
ester |
|
E |
I don’t have any ideas. |
|
Quickly add 65 cm3 of dilute sulfuric acid to the mixture.
What is the best piece of apparatus to use?
|
|
Anion |
Gas produced |
|
A |
carbonate |
carbon dioxide |
|
B |
iodide |
iodine |
|
C |
nitrate |
ammonia |
|
D |
sulfate |
sulfur dioxide |
|
E |
I don’t have any ideas. |
|