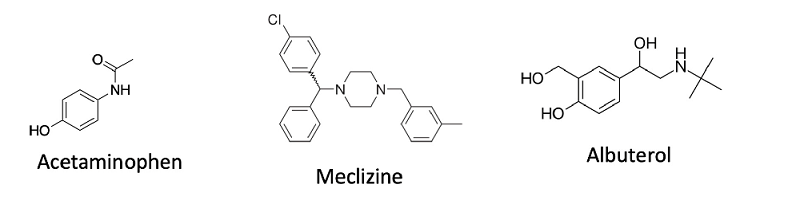
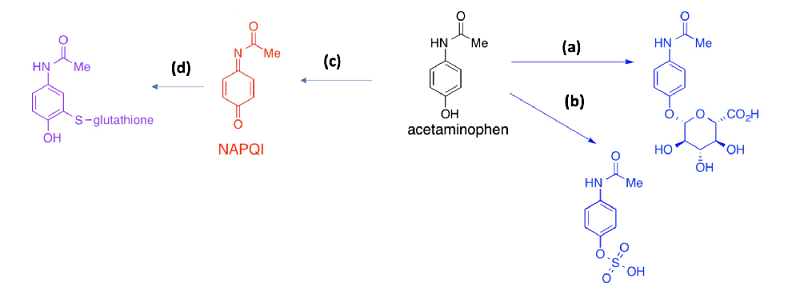
Medchem Quizzes
{"name":"Medchem Quizzes", "url":"https://www.quiz-maker.com/QPREVIEW","txt":"Test your knowledge of medicinal chemistry with our comprehensive quiz! This quiz contains 30 carefully crafted questions covering various aspects of drug interactions, solubility, metabolism, and more. Whether you're a student or a professional in the field, this quiz will challenge your understanding and provide valuable insights.30 Multiple Choice QuestionsInstant Feedback on AnswersExplore Key Concepts in Medicinal Chemistry","img":"https:/images/course6.png"}
More Quizzes
Potassium Compound
15814
Pharmacy council licence exam question with answer
271454
Tebakan cakra
320
Xxx
15821
Rent a Girlfriend - Which Character Are You?
201017362
Trig Identities - Free Practice Online
201017627
Am I a Negative Person? Free Personality
201017362
Am I Ready for My First Kiss? Boyfriend Kissing
201018121
High School Cliques - Which Group Are You In?
201016628
Words Ending in -ence and -ance - Free Spelling Test
201017834
Fingerprint Game - Test Your Matching Skills Free
201016628
Beer Trivia - 201+ Questions & Answers (Free)
201015346