Placement test A2 Level Chemistry V01
BrO3- + 5Br- + 6H+ → 3Br2 + 3H2O
The rate equation for the reaction is shown.
rate = k[BrO3-][Br-][H+]2
What are the units of the rate constant, k, for this reaction?
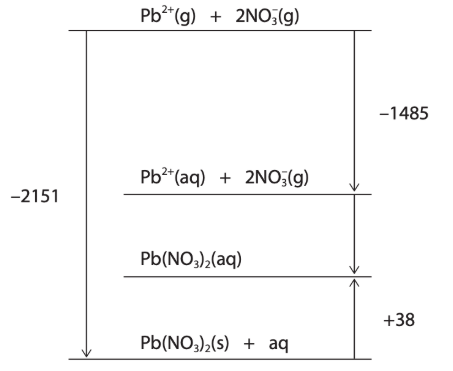
SiH4(g) + 2O2(g) → SiO2(s) + 2H2O(l)
Entropy data for this reaction are shown.
|
Species |
SiH4(g) |
O2(g) |
SiO2(s) |
H2O(l) |
|
So/ J K-1 mol-1 |
204.5 |
205.0 |
41.8 |
69.9 |
|
Iron |
Manganese |
|
|
A |
II → III |
VII → II |
|
B |
II → III |
VII → IV |
|
C |
III → II |
II → VII |
|
D |
III → II |
III → IV |
|
E |
I don’t have any ideas. |
|
C6H5OH + 3Br2 → C6H2Br3OH + 3HBr
[Mr values: C6H5OH = 94.0 Br2 = 159.8 C6H2Br3OH = 330.7 HBr = 80.9]
When 5.00 g of phenol was reacted and purified, the percentage yield of 2,4,6‑tribromophenol was 76.8%. What mass of purified 2,4,6‑tribromophenol was formed?
Zn2+(aq) + 2e- → Zn(s) Eo = -0.76 V
Ni2+(aq) + 2e- → Ni(s) Eo = -0.26 V
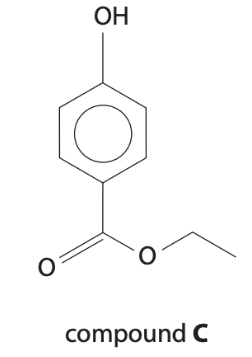
Compound C is chlorinated and hydrolysed and then the CO2 group is removed (decarboxylation) to form 2,6‑dichlorophenol. Other than phenol, name the fun