Oxygen atom model 3D quiz: structure and electron configuration
Quick quiz to test oxygen electron configuration and atomic structure. Instant results.
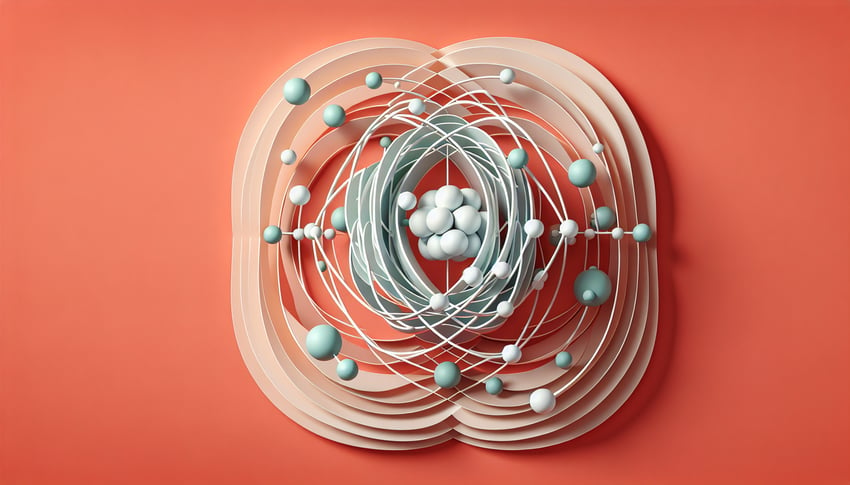
This quiz helps you read a 3D atomic model of oxygen, spot protons, neutrons, and electrons, and confirm its electron configuration. Use it to review fast before exams and fix weak spots. For more practice, check the atomic structure quiz, try an electron configuration test, or explore orbitals with an orbital notation quiz.
Study Outcomes
- Identify key components of the 3D atomic model of oxygen -
Learn to recognize and label the nucleus, electron shells, and subatomic particles that define oxygen's atomic structure through detailed 3D visualization.
- Analyze the spatial arrangement of electrons in oxygen's 3D atomic model -
Examine how electrons occupy specific energy levels and electron shells to understand oxygen's electron configuration in three dimensions.
- Distinguish between protons, neutrons, and electrons in the atomic structure quiz -
Develop the ability to differentiate each subatomic particle based on charge, mass, and position within the oxygen atom.
- Apply knowledge to excel at the parts of atom quiz -
Use your understanding of atomic structure to accurately identify and label each part of the oxygen atom under quiz conditions.
- Interpret detailed 3D visuals in the 3D atomic model quiz to reinforce concepts -
Enhance your comprehension of atomic theory by engaging with interactive 3D models that depict particle interactions and structural relationships.
- Evaluate performance on the subatomic particles quiz to target areas for improvement -
Review your quiz results to pinpoint strengths and weaknesses in understanding the roles and properties of protons, neutrons, and electrons.
Cheat Sheet
- Atomic Number and Protons -
In the 3d atomic model of oxygen, the atomic number (Z) is 8, which directly equals its number of protons. Since protons define the element identity (IUPAC), oxygen always has 8 positively charged particles in its nucleus. A simple mnemonic is "Z = Proton Power" to recall that Z gives the proton count.
- Mass Number and Neutrons -
The mass number (A) of the most common oxygen isotope is 16, so neutrons = A - Z = 16 - 8 = 8, giving oxygen-16 eight neutral particles in the nucleus. This subtraction formula is essential when tackling parts of atom quiz questions on isotopes. Remember "A minus Z stays neutrons" to lock in the calculation.
- Electron Configuration -
Oxygen's electron configuration is 1s² 2s² 2p❴, which fills the first shell with 2 electrons and places 6 in the second shell out of a possible 8. This distribution explains oxygen's - 2 oxidation state tendency in many compounds (e.g., H₂O). To memorize, use the phrase "1, 2, then p-four" when practicing for your atomic structure quiz.
- 3D Orbital Shapes -
In a 3d atomic model quiz context, oxygen's valence electrons occupy dumbbell-shaped 2p orbitals aligned along the x, y, and z axes. Quantum mechanical models (MIT OCW) depict these as probability clouds rather than fixed paths from the classical Bohr model. Visualize p orbitals as three perpendicular "peanuts" around the nucleus to ace your subatomic particles quiz.
- Interactive Practice Tools -
Engaging with a 3d atomic model quiz tool, like PhET's "Build an Atom," helps reinforce recognition of protons, neutrons, and electrons in real time. Frequent self-testing with parts of atom quiz modules boosts retention and confidence, turning abstract quantum concepts into tangible learning. Aim to score consistently above 80% to ensure mastery of subatomic fundamentals.







