BIO PHAY1003
Outline cell theory
All living organisms are composed of one or more units called cells
Each cell is capable of maintaining its own vitality independently
Cells can only arise form other cells
Viruses are not cells.
Viruses are cells
Cells reproduce by way of binary fission
Cells contain DNA, found specifically in the chromosome and RNA found in the cell nucleus & cytoplasm
All cells are basically the same in chemical composition in organisms of similar species
Size of viruses
20-400 nm
1-10 micrometres
10-30 micrometres
1000nm
10-100 micrometres
Prokaryotes
Large sizes due to central vacuole (responsible for growth)
Limited by efficient metabolism
Limited by surface area to volume ratio
Eukaryotic animal cells
Large sizes due to central vacuole (responsible for growth)
Limited by efficient metabolism
Limited by surface area to volume ratio
Plant cells (eukaryotic)
Large sizes due to central vacuole (responsible for growth)
Limited by efficient metabolism
Limited by surface area to volume ratio
What are viruses/virions...
Tiny particles visible through electron microscope
Tiny particles visible through light microscope
Cells
Contain genetic information in the form of DNA/RNA
Contain protein coat (capsid) which surrounds/protects genetic material
Non-infectious agent
In some cases envelope of lipids surrounds capsid when virus is outside of cell
Are cells because they carry genetic material, reproduce, & evolve through natural selection
Are not cells because they lack key characteristics (such as cell structure)
Viruses do not contain their own
Taking over host cell/infection
Cell to burst/be destroyed
Taking over cell machinery to synthesize new virus particles
Vaccines
Immune response
Biochemical machinery
Viruses only replicate by
Taking over host cell/infection
Cell to burst/be destroyed
Taking over cell machinery to synthesize new virus particles
Vaccines
Immune response
Biochemical machinery
Viruses multiply by
Taking over host cell/infection
Cell to burst/be destroyed
Taking over cell machinery to synthesize new virus particles
Vaccines
Immune response
Biochemical machinery
Virus particles released from the cell can cause
Taking over host cell/infection
Cell to burst/be destroyed
Taking over cell machinery to synthesize new virus particles
Vaccines
Immune response
Biochemical machinery
Viral infections in animals may cause
Taking over host cell/infection
Cell to burst/be destroyed
Taking over cell machinery to synthesize new virus particles
Vaccines
Immune response
Biochemical machinery
Artificial immunity can be induced by
Taking over host cell/infection
Cell to burst/be destroyed
Taking over cell machinery to synthesize new virus particles
Vaccines
Immune response
Biochemical machinery
Viruses structures
Caspid
Capsomere
Flagella
Lipid envelope
DNA/RNA
Mitochondria
Nucleus
Glycoprotein
Tail sheath/fibre
Cell wall
Capsid
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Capsomere
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Glycoprotein
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Nucleus
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Cell wall bacterial
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Golgi apparatus
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Endoplasmic Reticulum (rough)
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Endoplasmic Reticulum (smooth)
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Mitochondria
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Chloroplasts
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Vacuole
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Cell membrane
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Lysosome
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Cytoplasm
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Centrioles
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Plasma membrane (animal)
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Ribosomes
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Cell wall prokaryotes
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Glycocalyx
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Plasmid
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Bacterial chromosome/nucleoid
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Fimbriae
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Inclusion/granule
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Endospore
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Actin cytoskeleton
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Flagellum
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Outer membrane (bacteria)
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
LPS
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Porins
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Drug pumps
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Peroxisomes
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Cytoskeleton
Double stranded DNA circle containing extra genes
Continuous with outer membrane of nucleus, smooth without ribosomes involved in lipid metabolism.
structural integrity. Porous peptidoglycan = rigidity and cell shape
Stored nutrients (fat/phosphate/glycogen) deposited in dense crystals/particles
Continuous with outer mem of nucleus. Outer face studded with ribosomes. Engaged in protein synthesis.
Framework of tubular proteins giving animal cell shape and provides basis for movement
Two centrioles make up centrosome to which cell's cytoskeleton og microtubules is attached
Made of condensed DNA moleclues. DNA directs all genetics and heredity of cell and codes for all proteins
Thin sheet of lipid and protein surrounding cytoplasm and controls flow of materials in/out
Lipopolysaccharide. Highly branched fatty sugar. Acts as endotoxin as it induces fever and shock in human host.
Protect cell from internal turgor pressure
Power plant. Matrix has enz which break C-C bonds of food molecules. Energy transferred to ATP @ inner mitochondrial membrane.
Double layer of phospholipid bilayer that acts as semiipermeable barrier in which proteins are embedded (act as pumps/channels)
Tiny particles composed of protein and RNA that are used as sites for protein synthesis
Fine hairlike bristles from cell surface. Adhesion to other cells/surfaces
Pink coating external to cell. protective/adhesive/receptor functions
Specialized appnedage attached by basal body holding long rotating filament. Movement pushes cell forward (motility)
Vesicles containing hydrolytic enz that break down proteins lipids and nucleic acids. Breakdown of materials taken into cell/parts of cell being replaced
Class of protiens with carbohydrate groups attached to polypeptide chain
Water based solution filling entire cell
Dormant body formed within some that allows for survival in adverse conditions
Long fibres of proteins encircling cel inside cell mem. And contribute to shape
Similar structure to lysosomes. Produce and destroy hydrogen peroxide with oxidative enz. Away from rest of cell/damage
Extra membrane similar to cell membrane but also containing Lipopoly saccharide. LPS. Control sflow of materials and portions of it are toxic to mammals when released. Phospholipid membrane with tiny holes (porins)
Series of flattened sacs receiving vesicles from ER to modify sort and package contents and secrete to other organelles
In Gram -ve outer membrane. Block entrance of harmful chemicals and antibiotics making it more difficult to treat.
In many antibiotic resistant bacteria. Connected to porins. They pump out any drugs/harmful chemicals that enter.
Self-assembling subunit of a capsid
Protein coat protecting genetic information
Genetic material DNA held in chromatin fibres. Nucleolus assembles cell's ribosomes and communicates with cytoplasm via pores in double layered nuclear envelope
Light microscope
General outline of cells
General outline of cell structure
Internal architecture of cells
Limited by wavelength of visible light (400-700 nm)
Improve resolution with radiation of longer wavelength
Beam of electrons as short as 5 picometres using very high voltages
Improve resolution with radiation of shorter wavelength
Beam focused using electrostatic/magnetic lenses
Highest magnification achievable is approx. 1500
Highest magnification achievable is approx. 500,000
Cells are
Surrounded by a plasma membrane with a viscous fluid (cytoplasm)
Eg bacteria
Units of which bodies of living organisms are made
Plants/animal clels
Every living cell is
Surrounded by a plasma membrane with a viscous fluid (cytoplasm)
Eg bacteria
Units of which bodies of living organisms are made
Plants/animal clels
Prokaryotic cells are
Surrounded by a plasma membrane with a viscous fluid (cytoplasm)
Eg bacteria
Units of which bodies of living organisms are made
Plants/animal clels
Eukaryotic cells are
Surrounded by a plasma membrane with a viscous fluid (cytoplasm)
Eg bacteria
Units of which bodies of living organisms are made
Plants/animal clels
Eukaryotic cells are simpler in their structure than prokaryotic cells
True
False
Prokaryotic cells
No nuclear envelope
Nuclear envelope
Chemically complex cell wall
Cell wall only in plants/fungi (simple)
Larger cell size
Lack certain intracellular organelles
Contain organelles eg mitochondria
Small cell size
Two or more chromosomes
Histones bound to DNA in nucleus
No nucleolus
Lack microtubules, microfilaments
No histone proteins with DNA in nucleus
One chromosome
One or more nucleoli
Contain microtubules, microfilaments
Bacteria
Viruses
1 - 10 micrometres
10 - 30 micrometres
10 - 100 micrometres
Bacteria cells major shapes
Bacillus (coils) spirilla (round) coccus (coils)
Coccus (round) bacillus (rods) spirilla (coils
Bacillus (round) spirilla (coils) coccus (rods)
Spirilla (round) coccus (rods) bacillus (coils)
Bacterial cell structure: external
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Bacterial cell structure: internal organelles
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Appendages for
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Bacterial cell structure: cell envelope
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Adhesion by
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Movement by
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Cell wall by
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Peptidoglycan by
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Glycan chains
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Antibiotics stop bacterial infections by
Long glycan sugar chains cross-linked by peptides
Chromosome/ribosome
NAG or NAM
Cell wall and membranes
Flagella
Movement and adhesion
Peptidoglycan
Pili, fimbriae, and glycocalyx
Interfering with cell wall synthesis (not harming human cells)
Appendages and coverings
Gram +ve
Type of cell
Type of bacteria
Type of virus
Thick cell wall
Thin cell wall
Stains pink with crystal violet dye
Stains purple with crystal violet dye
Contains teichoic acids
Does not contain teichoic acids
20 - 80nm peptidoglycan
1-3nm peptidoglycan
Require extra layer of protection
Gram -ve
Thick cell wall
Thin cell wall
20 - 80 nm peptidoglycan
1 - 3 nm peptidoglycan
Stains purple with crystal violet dye
Stains pink with crystal violet dye
Require extra layer of protection: outer membrane
Contains teichoic acid
Does not contain teichoic acids
Eukaryotic cells
Basic units of animals and plants
Not single celled types and fungi
And single celled types & fungi
Plant cells generally have large
Proteins and other lipids
Double layer of phospholipid
Chlorophyll for photosynthesis to trap sunlight => carbs.
Fluid filled vacuoles.
Cell walls. Animal cells do not.
Dynamic structures controlling contents of compartments they enclose
Plant cells have
Proteins and other lipids
Double layer of phospholipid
Chlorophyll for photosynthesis to trap sunlight => carbs.
Fluid filled vacuoles.
Cell walls. Animal cells do not.
Dynamic structures controlling contents of compartments they enclose
Plant cells have chloroplasts that contain
Proteins and other lipids
Double layer of phospholipid
Chlorophyll for photosynthesis to trap sunlight => carbs.
Fluid filled vacuoles.
Cell walls. Animal cells do not.
Dynamic structures controlling contents of compartments they enclose
Cell membranes consist of a
Proteins and other lipids
Double layer of phospholipid
Chlorophyll for photosynthesis to trap sunlight => carbs.
Fluid filled vacuoles.
Cell walls. Animal cells do not.
Dynamic structures controlling contents of compartments they enclose
Embedded in fluid mosaic are
Proteins and other lipids
Double layer of phospholipid
Chlorophyll for photosynthesis to trap sunlight => carbs.
Fluid filled vacuoles.
Cell walls. Animal cells do not.
Dynamic structures controlling contents of compartments they enclose
Membranes are not passive barriers but
Proteins and other lipids
Double layer of phospholipid
Chlorophyll for photosynthesis to trap sunlight => carbs.
Fluid filled vacuoles.
Cell walls. Animal cells do not.
Dynamic structures controlling contents of compartments they enclose
Plasma membrane
Same as outer membrane
Controls passage of materials into and out of cell
Receives messages that regulate behaviour of cell
Single layer phospholipid
ER
3D network of membranes extending throughout cell
Rough: covered on cytoplasmic side with ...
Large surface area
Site of protein synthesis
All of the above
Golgi apparatus
3D network of membranes
Site of protein synthesis
Specialised region of membranes enclosing cisternae from which vesicles arise
Carbs added to proteins coming from ER to form glycoproteins
Where glycoproteins secreted at cell surface/sent to other parts
Where parts are rough or smooth
Small surface area
Mitochondria
Small elongated bodies
Electron micrograph: internal structure
Seen clearly through light microscope
Internal space/matrix has enzymes and some DNA
Produce glucose
Produce ATP to carry energy to processes in cell
Useful for muscle contraction/active transport/protein synthesis
Largest organelle within cell
Nucleus
Largest of organelles
Contains chloroplasts
Is where protein synthesis takes place
Not present in most cells
Found in prokaryotic cells
Encloses nuclear membrane
Double membrane surrounds it with pores (seen via electron micrograph)
Contains DNA attached to histones => chromatin
More densely packed chromatin granules appear as
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
During cell division the chromatin granules form
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Chloroplasts found only in
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Matrix of plant cell is the
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Cell wall is a non-living layer not found in
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Stroma has stacks of _______________ which contain chlorophyll
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Green pigment for light trapping belongs to
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Cell wall is derived from
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Cellulose is a
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
A network of various sorts of protein filaments in cytoplasm
Animal cells
Glucose polymer
Nucleoli
Chromosomes
Lamellae (grana)
Stroma
Chlorophyll
Plant cells
Cytoskeleton
Cellulose
Groups of cells are organised into
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Tissue examples:
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Tissues are organised into
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Organs may be organized into
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Organ systems
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Cell
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Tissue
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Organ
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Organ system
Organs
Tissues
Self-contained part of organism (group of tissues) with a specific vital function
Respiratory, immune, circulatory, digestive
Large mass if specialized cells and their products making up part of an organism for a specific function
Smallest structural and functional unit of an organism
Organ systems
Group of organs that work together to perform one or more function
Epithelial, muscle, connective and nervous
Mitosis
Two identical daughter cells
Different from parent cells
DNA with histone proteins can be seen as threads = 'chromosomes'
Somatic cell division for growth/produce cells during repair/replacement
Produces half number of chromosomes as original
Produce gametes (Haploids)
4 main stages: Metaphas Interphase Anaphase Prophase
Before division a cell is
Merged, during the actual active process
Not visible
A granular body with one or more dense nucleoli
In interphase
Chromosomes in interphase are
Merged, during the actual active process
Not visible
A granular body with one or more dense nucleoli
In interphase
In interphase nucleus appears as
Merged, during the actual active process
Not visible
A granular body with one or more dense nucleoli
In interphase
Mitosis has stages which are
Merged, during the actual active process
Not visible
A granular body with one or more dense nucleoli
In interphase
Prophase
Nuclear membrane disappears
Spindle microtubules shorten and drag chromatids away from equator
Groups of chromatids assemble at the poles
Nucleolus disappears gradually
Centrioles are duplicated
Chromosomes seen as long thin entangled threads in nuclear membrane
Chromatids move to opposite poles of cell
Threads become shorter and thicker
Spindle made from microtubules begins to form
DNA content is doubled
Chromatids become uncoiled
Centrioles migrate to opp ends of cell
Visible two chromatids joined at the centromere
Centromere of chromosome splits and moves to opp poles
Cell membrane between two nuclei invaginates
Chromosomes become attached by centromeres to equator of spindle
Nucleoli reform and nucleus takes on granular appearance
Cytoplasmic cleavage occurs (cytokinesis)
Two daughter cells (copies of original) are formed
Nuclear membrane forms around chromatids at poles
Four daughter cells (copies of original) are formed
Each chromosome seen as 2 chromatids
Metaphase
Nuclear membrane disappears
Chromosomes seen as long thin entangled threads in nuclear membrane
Spindle microtubules shorten and drag chromatids away from equator
Groups of chromatids assemble at the poles
Threads become shorter and thicker
Nucleolus disappears gradually
Centrioles are duplicated
Centrioles migrate to opp ends of cell
DNA content is doubled
Spindle made from microtubules begins to form
Chromatids become uncoiled
Visible two chromatids joined at the centromere
Chromatids move to opposite poles of cell
Nucleoli reform and nucleus takes on granular appearance
Chromosomes become attached by centromeres to equator of spindle
Centromere of chromosome splits and moves to opp poles
Nuclear membrane forms around chromatids at poles
Two daughter cells (copies of original) are formed
Cytoplasmic cleavage occurs (cytokinesis)
Four daughter cells (copies of original) are formed
Cell membrane between two nuclei invaginates
Each chromosome seen as 2 chromatids
Anaphase
Nuclear membrane disappears
Chromosomes seen as long thin entangled threads in nuclear membrane
Spindle microtubules shorten and drag chromatids away from equator
Nucleolus disappears gradually
Groups of chromatids assemble at the poles
Chromatids move to opposite poles of cell
Centrioles are duplicated
Threads become shorter and thicker
Spindle made from microtubules begins to form
DNA content is doubled
Nuclear membrane forms around chromatids at poles
Visible two chromatids joined at the centromere
Chromosomes become attached by centromeres to equator of spindle
Two daughter cells (copies of original) are formed
Centromere of chromosome splits and moves to opp poles
Centrioles migrate to opp ends of cell
Chromatids become uncoiled
Nucleoli reform and nucleus takes on granular appearance
Cytoplasmic cleavage occurs (cytokinesis)
Four daughter cells (copies of original) are formed
Cell membrane between two nuclei invaginates
Telophase
Groups of chromatids assemble at the poles
Nuclear membrane disappears
Chromosomes seen as long thin entangled threads in nuclear membrane
Threads become shorter and thicker
Nucleolus disappears gradually
Spindle microtubules shorten and drag chromatids away from equator
Centrioles are duplicated
Centrioles migrate to opp ends of cell
Chromatids move to opposite poles of cell
Spindle made from microtubules begins to form
DNA content is doubled
Visible two chromatids joined at the centromere
Chromatids become uncoiled
Cell membrane between two nuclei invaginates
Chromosomes become attached by centromeres to equator of spindle
Centromere of chromosome splits and moves to opp poles
Nuclear membrane forms around chromatids at poles
Nucleoli reform and nucleus takes on granular appearance
Four daughter cells (copies of original) are formed
Cytoplasmic cleavage occurs (cytokinesis)
Two daughter cells (copies of original) are formed
Cell cycle
Largest part of cell cycle
2. Replication of DNA completed and duplication of histone proteins
Total process of growht and division by mitosis
3. Synthesis of organelles such as mitochondria
1. Synthesis of RNA and protein
Interphase
Largest part of cell cycle
2. Replication of DNA completed and duplication of histone proteins
Total process of growht and division by mitosis
3. Synthesis of organelles such as mitochondria
1. Synthesis of RNA and protein
G1 stage
Largest part of cell cycle
2. Replication of DNA completed and duplication of histone proteins
Total process of growht and division by mitosis
3. Synthesis of organelles such as mitochondria
1. Synthesis of RNA and protein
G2 stage
Largest part of cell cycle
2. Replication of DNA completed and duplication of histone proteins
Total process of growht and division by mitosis
3. Synthesis of organelles such as mitochondria
1. Synthesis of RNA and protein
S stage
Largest part of cell cycle
2. Replication of DNA completed and duplication of histone proteins
Total process of growht and division by mitosis
3. Synthesis of organelles such as mitochondria
1. Synthesis of RNA and protein
Cell cycle
0%
0
0%
0
0%
0
Meiosis
Organisms that reproduce sexually to produce gametes
One nuclear division
Two nuclear divisions
Forms diploid cells
Forms haploid cells/gametes
In meiosis prophase 1
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Each bivalent is
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Gradually chromosomes shorten and thicken, seen to be composed of
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Crosslinks called ________ develop between chromosomes and their homologous partner
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Nucleoli disappear and spindle laid down in cytoplasm by
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Formulation of crosslinks between chromatids leads to
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Recombination is when
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Because of crossing over,
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Karyotype is
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Genes further apart on the chromosome,
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Recombination is basis of a method of
A homologous pair of chromosomes
Centrioles, that divided @ interphase
Two chromatids
Portions of genetic material exchanged leading to shuffling of genes within the homologous pairs
The number and appearance of chromosomes in the nucleus of a eukaryotic cell
Chiasmata
Linked genes (same chromosome), may be separated
Chromosome mapping by linkage analysis
More likely to be separated. Vice versa.
Recombination
Chromosomes pair up to form bivalents in nuclear envelope
Metaphase I
Spindle microtubules shorten and drag homologous chromosomes of each bivalent apart to opp poles
Nuclear membrane breaks down
Chromosomes surrounded by new nuclear membrane
Nucleoli reappear
2 groups of chromosomes assemble at opp poles
Bivalents (still arranged as homologous pairs) attach by centromeres to microtubules at spindle equator
Centromeres of chromosomes divide and chromatids pulled to opp poles
Nuclei take on granular appearance
Chromosomes uncoil, nucleoli reappear
Chromatids assemble at poles and are surrounded by nuclear membranes.
Nucleoli reappear, spindle disappear and cleavage of cytoplasm follows
Chromosomes appear in both nuclei
Orientation of bivalents is random; bases of further shuffling of genes by reassortment
Cytoplasmic cleavage may occur followed by short interphase (no DNA replication)
No pairing of chromosomes and no crossing over
Resulting cells are haploid; halved chromosome number
Centrioles move to opp ends of cell (90 deg. To first) and new spindle formed
Chromosomes seen to composed of 2 chromatids, orientation random (further reassortment)
Result is two new cells, each with same amount of DNA as diploid cell
Centrioles move to opp ends at right angles
Recombination/re-assortment, genetically not identical to parents
Nuclear membranes disappear and chromosomes attached by centromeres to microtubules at equator of spindle
Anaphase 1
Spindle microtubules shorten and drag homologous chromosomes of each bivalent apart to opp poles
Nuclear membrane breaks down
Chromosomes surrounded by new nuclear membrane
Nucleoli reappear
2 groups of chromosomes assemble at opp poles
Bivalents (still arranged as homologous pairs) attach by centromeres to microtubules at spindle equator
Centromeres of chromosomes divide and chromatids pulled to opp poles
Nuclei take on granular appearance
Chromosomes uncoil, nucleoli reappear
Chromatids assemble at poles and are surrounded by nuclear membranes.
Nucleoli reappear, spindle disappear and cleavage of cytoplasm follows
Chromosomes appear in both nuclei
Orientation of bivalents is random; bases of further shuffling of genes by reassortment
Cytoplasmic cleavage may occur followed by short interphase (no DNA replication)
No pairing of chromosomes and no crossing over
Resulting cells are haploid; halved chromosome number
Centrioles move to opp ends of cell (90 deg. To first) and new spindle formed
Chromosomes seen to composed of 2 chromatids, orientation random (further reassortment)
Result is two new cells, each with same amount of DNA as diploid cell
Centrioles move to opp ends at right angles
Recombination/re-assortment, genetically not identical to parents
Nuclear membranes disappear and chromosomes attached by centromeres to microtubules at equator of spindle
Telophase 1
Spindle microtubules shorten and drag homologous chromosomes of each bivalent apart to opp poles
Nuclear membrane breaks down
Chromosomes surrounded by new nuclear membrane
Nucleoli reappear
2 groups of chromosomes assemble at opp poles
Bivalents (still arranged as homologous pairs) attach by centromeres to microtubules at spindle equator
Centromeres of chromosomes divide and chromatids pulled to opp poles
Nuclei take on granular appearance
Chromosomes uncoil, nucleoli reappear
Chromatids assemble at poles and are surrounded by nuclear membranes.
Nucleoli reappear, spindle disappear and cleavage of cytoplasm follows
Chromosomes appear in both nuclei
Orientation of bivalents is random; bases of further shuffling of genes by reassortment
Cytoplasmic cleavage may occur followed by short interphase (no DNA replication)
No pairing of chromosomes and no crossing over
Resulting cells are haploid; halved chromosome number
Centrioles move to opp ends of cell (90 deg. To first) and new spindle formed
Chromosomes seen to composed of 2 chromatids, orientation random (further reassortment)
Result is two new cells, each with same amount of DNA as diploid cell
Centrioles move to opp ends at right angles
Recombination/re-assortment, genetically not identical to parents
Nuclear membranes disappear and chromosomes attached by centromeres to microtubules at equator of spindle
Prophase II
Spindle microtubules shorten and drag homologous chromosomes of each bivalent apart to opp poles
Nuclear membrane breaks down
Chromosomes surrounded by new nuclear membrane
Nucleoli reappear
2 groups of chromosomes assemble at opp poles
Bivalents (still arranged as homologous pairs) attach by centromeres to microtubules at spindle equator
Centromeres of chromosomes divide and chromatids pulled to opp poles
Nuclei take on granular appearance
Chromosomes uncoil, nucleoli reappear
Chromatids assemble at poles and are surrounded by nuclear membranes.
Nucleoli reappear, spindle disappear and cleavage of cytoplasm follows
Chromosomes appear in both nuclei
Orientation of bivalents is random; bases of further shuffling of genes by reassortment
Cytoplasmic cleavage may occur followed by short interphase (no DNA replication)
No pairing of chromosomes and no crossing over
Resulting cells are haploid; halved chromosome number
Centrioles move to opp ends of cell (90 deg. To first) and new spindle formed
Chromosomes seen to composed of 2 chromatids, orientation random (further reassortment)
Result is two new cells, each with same amount of DNA as diploid cell
Centrioles move to opp ends at right angles
Recombination/re-assortment, genetically not identical to parents
Nuclear membranes disappear and chromosomes attached by centromeres to microtubules at equator of spindle
Metaphase II
Spindle microtubules shorten and drag homologous chromosomes of each bivalent apart to opp poles
Nuclear membrane breaks down
Chromosomes surrounded by new nuclear membrane
Nucleoli reappear
2 groups of chromosomes assemble at opp poles
Bivalents (still arranged as homologous pairs) attach by centromeres to microtubules at spindle equator
Centromeres of chromosomes divide and chromatids pulled to opp poles
Nuclei take on granular appearance
Chromosomes uncoil, nucleoli reappear
Chromatids assemble at poles and are surrounded by nuclear membranes.
Nucleoli reappear, spindle disappear and cleavage of cytoplasm follows
Chromosomes appear in both nuclei
Orientation of bivalents is random; bases of further shuffling of genes by reassortment
Cytoplasmic cleavage may occur followed by short interphase (no DNA replication)
No pairing of chromosomes and no crossing over
Resulting cells are haploid; halved chromosome number
Centrioles move to opp ends of cell (90 deg. To first) and new spindle formed
Chromosomes seen to composed of 2 chromatids, orientation random (further reassortment)
Result is two new cells, each with same amount of DNA as diploid cell
Centrioles move to opp ends at right angles
Recombination/re-assortment, genetically not identical to parents
Nuclear membranes disappear and chromosomes attached by centromeres to microtubules at equator of spindle
Anaphase II
Spindle microtubules shorten and drag homologous chromosomes of each bivalent apart to opp poles
Nuclear membrane breaks down
Chromosomes surrounded by new nuclear membrane
Nucleoli reappear
2 groups of chromosomes assemble at opp poles
Bivalents (still arranged as homologous pairs) attach by centromeres to microtubules at spindle equator
Centromeres of chromosomes divide and chromatids pulled to opp poles
Nuclei take on granular appearance
Chromosomes uncoil, nucleoli reappear
Chromatids assemble at poles and are surrounded by nuclear membranes.
Nucleoli reappear, spindle disappear and cleavage of cytoplasm follows
Chromosomes appear in both nuclei
Orientation of bivalents is random; bases of further shuffling of genes by reassortment
Cytoplasmic cleavage may occur followed by short interphase (no DNA replication)
No pairing of chromosomes and no crossing over
Resulting cells are haploid; halved chromosome number
Centrioles move to opp ends of cell (90 deg. To first) and new spindle formed
Chromosomes seen to composed of 2 chromatids, orientation random (further reassortment)
Result is two new cells, each with same amount of DNA as diploid cell
Centrioles move to opp ends at right angles
Recombination/re-assortment, genetically not identical to parents
Nuclear membranes disappear and chromosomes attached by centromeres to microtubules at equator of spindle
Telophase II
Spindle microtubules shorten and drag homologous chromosomes of each bivalent apart to opp poles
Nuclear membrane breaks down
Chromosomes surrounded by new nuclear membrane
Nucleoli reappear
2 groups of chromosomes assemble at opp poles
Bivalents (still arranged as homologous pairs) attach by centromeres to microtubules at spindle equator
Centromeres of chromosomes divide and chromatids pulled to opp poles
Nuclei take on granular appearance
Chromosomes uncoil, nucleoli reappear
Chromatids assemble at poles and are surrounded by nuclear membranes.
Nucleoli reappear, spindle disappear and cleavage of cytoplasm follows
Chromosomes appear in both nuclei
Orientation of bivalents is random; bases of further shuffling of genes by reassortment
Cytoplasmic cleavage may occur followed by short interphase (no DNA replication)
No pairing of chromosomes and no crossing over
Resulting cells are haploid; halved chromosome number
Centrioles move to opp ends of cell (90 deg. To first) and new spindle formed
Chromosomes seen to composed of 2 chromatids, orientation random (further reassortment)
Result is two new cells, each with same amount of DNA as diploid cell
Centrioles move to opp ends at right angles
Recombination/re-assortment, genetically not identical to parents
Nuclear membranes disappear and chromosomes attached by centromeres to microtubules at equator of spindle
Significance of meiosis
Cells produced are gametes
@ fertilisation, form zygote; 2 haploid cells fuse to form once cell
In sexual reproduction resulting organism exactly the same as parents
Cells produced diploids
Gametes have varied combination due to reassortment/recombination
Gametes have varied combination due to linkage analysis
Draw simple diagrams of typical viruses, bacteria, plant cells and animal cells showing the structure as revealed by electron microscopy, including; nucleus, mitochondria, chloroplasts, endoplasmic reticulum, Golgi apparatus, ribosomes, lysosomes, peroxisomes, plasma membrane, vacuoles, cell wall and cytoplasm.
List at least five characteristics which could be used to distinguish prokaryotic and eukaryotic organisms.
Describe the process of cell division by mitosis in terms of the major events that occur in interphase, prophase, metaphase, anaphase and telophase.
Show how the cell cycle can be divided into G1 phase, S phase, G2 phase, mitosis and cytoplasmic cleavage and represented as a pie-chart.
Describe the major events of meiosis in terms of prophase I, metaphase I, anaphase I, telophase I, followed by prophase II, metaphase II, anaphase II and telophase II.
Glycogen breakdown.
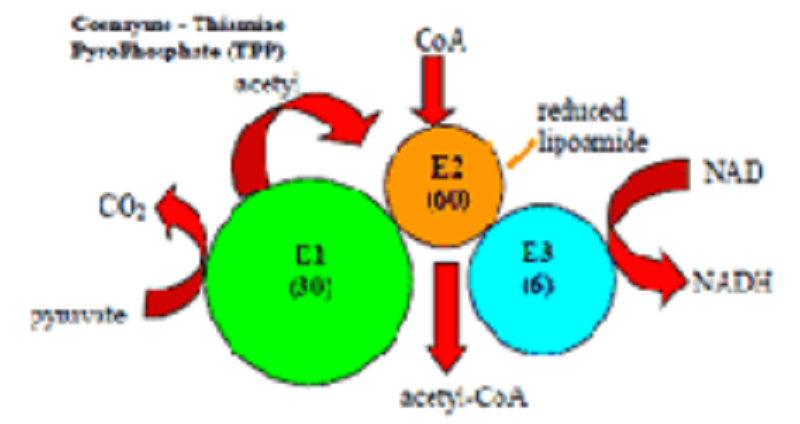
Pyruvate decarboxylase
Dihydrolipoyl dehydrogenase
Transfers acetyl group to CoA
Uses coenzyme lipoamide
Transfers acetyl group to CoA
Triacylglyceride
TCA cycle
Trasacetylase
Removes CO2 from pyruvate and oxidizes it
Vitamin B1
Pentose phosphate pathway
6-phosphogluconolactone
Pyruvate dehydrogenase
G6P dehydrogenase
ATP
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
NADPH
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Pentose phosphate pathway
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
In pentose phosphate pathway G-6-P is converted to
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Enzyme converting G-6-P in PPP is called ___________
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Enzyme G6P dehydrogenase reduces
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Acetyl CoA
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Pyruvate dehydrogenase complex consists of
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Mitochondrial membrane is impermeable to
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Because mit. mem. Is impermeable to _, acetyl-CoA forms __ in the first step of TCA cycle
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Citrate crosses the inner mitochondrial membrane on a _________ transporter
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
In the cytosol citrate is cleaved by _ to regenrate acetyl-CoA and oxaloacetate
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Oxaloacetate passes back into the mitochondria via
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Transfer of acetyl-CoA is at the expense of a molecule of
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
TAG synthesis is in
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
TAG synthesis is achieved by multienzyme complex
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
TAG synthesis has the multienzyme found to the
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
TAG multienzyme has 3 enzymes: glycerol-3-phosphate acyltransferase (GPAT), lysophosphatidate acyl transferase (LPAT) and
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
GPAT
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
LPAT
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
DGAT
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Glucose in excess of immediate energy requirements can be used to synthesize FA that are then _ to TAG
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Esterification of FA occurs mostly in ___ which can also remove dietary fats and FA in bloodstream
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Adipose tissue
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Fat in adipose cells is synthesised in the _ and then passed to the droplet
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Fat synthesised by liver is sent to adiposee tissue in _
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
In times of starvation TAG is hydrolysed (___________)
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Also in times of starvation free FA released into the blood for tissues to make energy from
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Human genetic make-up ensuring efficient use of substrates for energy
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
The thrifty genotype
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Many people of 21st century are having large supply of high calorie food storing excessive amounts of carbs and fat as
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
BMI
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
More obese, more prone to high bp, cardiovascular disease, _
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Food intake is controlled by neurons in _________ in brain
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Hypothalamus recognizes neural inputs and _____ about amount of food in digestive system
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Hormones released by adipose tissue
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Hormones released by stommach and adipose tissue signal hypothalamus to adjust feeding according to how much in stomach and how much stored in
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
Drug companies interested in mimicking hormonal effects to _________ appetite
Diabetes mellitus
Leptin
ATP-Citrate lyase
Cytosolic side of ER
Malate and pyruvate
Tricarboxylic acid
Esterified
Diacylglycerol acyl transferase (DGAT)
ATP
Adipose tissue (stored) or in liver from which its taken to adipose/other tissues
NADP to NADPH
TAG in adipose tissue
Esterifies a saturated FA to make TAG
Adipocytes
6-phosphogluconolactone
G6P dehydrogenase
Triacylglycerol synthetase
Esterifies a saturated FA to make monoacylglycerol
Esterifies an unsaturated FA to make diacylglycerol
Citrate
In cytosol by pentos phosphate pathway
Liver and adipose tissue
Adipocytes adapted for fat storage with thin peripheral strip of cytosol surroudning large droplet of TAG
Cytosol
Mitochondria- ox. Phosphorylation and transfeerred via transporter to cytosol
Lipolysis
B-oxidation
'the thrifty genotype'
Genes that code for proteins (enz) that are good at storing fat in adipose tissue
Mitochondria- multienzyme complex pyruvate dehydrogenase
Sympathetic nerve impulses
E1, E2, E3 acting in sequence in a mini pathway
Hypothalamus
Lipoproteins
CoA derivatives
Short pathway on side of glycolysis
Suppress
Body weight (kg) / Height^2 (m)
What to kinases do
GDP/GTP?
Energy molecules
Guanosine triphosphate and guanosine diphosphate
Used in glycolysis
Acetyl-CoA forms __________ in the first step of the ____________ cycle and it then crosses the inner mitochondrial membrane on a __________________ transporter
Oxidative phosphorylation
TCA
Tricarboxylic acid
Krebs
Citrate
Phosphogluconomutase
Oxaloacetate
ATP-Citrate lyase
Acetate
Thiamine Pyrophosphate
Glycogen-6-Phosphate
Malate
Pyruvate
In the cytosol the ___________ is cleaved by ___________ to regenerate acetyl-CoA (for fatty acid synthesis) and _________________(which passes back into mitochondria via _______________ and ____________________)
Oxidative phosphorylation
TCA
Tricarboxylic acid
Krebs
Citrate
Phosphogluconomutase
Oxaloacetate
ATP-Citrate lyase
Acetate
Thiamine Pyrophosphate
Glycogen-6-Phosphate
Malate
Pyruvate
Acetyl-coA Carboxylase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Pyruvate dehydrogenase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Pyruvate decarboxylase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Hexokinase/glucokinase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Phosphoglucomutase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Dihydrolipoyl dehydrogenase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Lipoamide coenzyme
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Thiamine pyrophosphate coenzyme
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
G-1-P uridylyl transferase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Orthophosphate
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Inorganic pyrophosphatase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Debranching enzyme
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Branching enzyme
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Glycogenin
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Glycogen synthase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Glycogen phosphorylase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Glucose - 6- phosphatase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Fatty acid synthase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
ACC
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
ACP
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Phosphopantetheine coenz
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Nucleoside diphosphatase
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Why not use G-1-P instead of UDP glucose for glycogen synthesis?
PDH multienzyme complex with E1 E2 and E3 uses pyruvate to form Acetyl-CoA necessary for fatty acid synthesis
Join 2C units of Acetyl CoA to form C-C-C-C-C(n( chain of fatty acids. It is activated by carboxylating acetyl CoA to malonyl CoA (uses ATP). Regulatory enzyme determining FA synthesis rate
Primer, short oligosaccharide which is a protein composed of a-1,4 glucose units attached to the phenolic oxygen atom of a Tyr AA contained in primary structure
Clucose resdues added from UDP-G and joined by a-1,4 links (originally attached to molecule of glycogenin)
Carries out cycle of rxns where long saturated fatty acid (C16 palmitate) made by successive condensation reactions of 2-C units derived form £-C malonyl-CoA
Converts glucose-1-phosphate to glucose-6- phosphate.
A-1,6 glucosidase hydrolyses a-1,6 linkage (glycogen breakdown)
Long flexible conenz attached to serine (then ACP). Successive condensation of 2-C units occurs linked to sulphydryl terminus of this prosthetic group.
Phosphorolysis: Glycogen (n) to G-1-P + Glycogen (n-1) reversible in vitro but irreversible invitro due to [Pi] > glucose-1-phosphate
G-6-P to glucose since G6P cannot diffuse out of cells.
Acyl-CoA carboxylase irreversible catalysis of Acetyl-CoA to activate its 2-C subunits, converted to malonyl-CoA using ATP. Regulatory enzyme.
Amylo-4,6 transferase: when chain is at least 11 residues in length 6-8 residue block cleaved by this enz, breaks a-1,4 link, and reattaches to main chain via a-1,6 link, new branch point being 4 residues away from pre-existing.
Acyl carreir protein. Bound to a serine is the long, flexible phosphopantetheine coenzyme
UDP to UTP using ATP
Activated state, UDP is a better leaving group than phosphate
Glycogen storage diseases
Type V
McArdle's disease
Muscle unable to perform strenuous exervise
Gets powerful muscle cramps and fatigue
Muscle has abnormal glycogen structure but increased in amount
Genetic defect is presence of glycogen phosphorylase; cannot breakdown G1P to feed into glycolysis via G6P => decreased eneregy production in muscle
Type VI, Hers' deisease; dangerous as patients have glycogen phosphorylase absent from liver. => liver enlargement due to increased glycogen an dhypoglycaemia as they cannot breakdown glycogen to G1P to G6P and release glucose in blood. During fasting; struggle to maintain blood glucose.
Macromolecules
Proteins
Nucleic acids
Minerals
Vitamins
Needed in large amounts
Carbs, fats, protein, wtaer
Provide structural material
AA from which proteins are built
Lipids from which cell mem are built
Fibre complex carb nondigestible (cellulose) needed for mechanical reasons (gut motility)
Ions, iron and sodium
Dietary fibre
Promotes gut motility/peristalsis
Insoluble fibre found in whole-wheat, nuts and veg
Soluble fibre in oats, peas, beans and fruis dissolves in water to make gel, slowing food movement
Help lower blood glucose as it slows sugar absorption. Reduce type 2 diabetes risk
Insoluble fibre in oats, peas beans and fruits dissolves in water to make gel, slowing food movement
Soluble fibre found in whole-wheat, nuts and veg
Molecule of dietary fat
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Saturated fats
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Unsaturated FA
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Unsaturated fats may be
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Dependent on C=C location, unsaturated fatty acids are
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Most FA synthesized by body but 2 acids are essential for diet:
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
EPA is also obtained from
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
AA also obtained from
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
EPA and AA are precursors of
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
EPA series less potent than those fro AA, and individuals are encouraged to
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
There is no aa storage provision so amino acids must
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
20 aa found in body and 10 synthesised whereas 10 are
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Sources of dietary protein include
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Excess aa oxidised for energy or converted to glucose (_______)
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Dietary minerals are
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Macrominerals
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Trace minerals required
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Trace mineral Cu required as
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Iodine is required for the biosynthesis of
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Iron is required for many enzymes, particularly for
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Vitamins recognizes as
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Vitamin C
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
L-ascorbic acid
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Cellular metabolism requires redox rxns, and damaging agents
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Free radicals must be neutralized by
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Oxidative stress
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Vitamin C is also a cofactor in at least 8 enzymatic rxns including several
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Collagen formation is important in
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Scurvy, lack of Vit C
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Scurvy leads to formation of brown spots on
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Pasteurisation (heat treated milk) controlled infant outbreaks of scurvy but
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Vitmain like compounds recommended like carnitine. Carnitine is
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Vitamin B1
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Thiamine is
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
TPP
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
PDH is part of generation of energy as ATP. In nervous system PDH also inovolved in
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Thiamine dependent enzymes present in all body cells, so B1 deficiency afect all organs sytems. however _ and _ particularly sensitive
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Insufficient Vit B1 leads to
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Dry beri beri
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Wet beriberi
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Peripheral neuropathy
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Phytochemicals present in food/plants
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Polyphenol antioxidants
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Phytochemical zeaxanthin
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Carotenoid, beta-cryptoxanthin appears to
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Lycopene
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Nutraceutical
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Nutraceutical products include isolated nutrients,
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Vitamin B complex supplement
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Vit b deficiency causes berberi includes
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Dietary supplement
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Dietary ingredients of dietary supplement include
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Nutraceuticals not as tightly regulated as _ and are available/monitored with same scrutiny as dietary supplements
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Dietary supplements can be extracts/concentrates found in forms like
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Use of broad based definitions creates inconsistent credibility distinguishing
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Entry of dietary nutrients into cell requires process of ____ into bloodstream for transport to needy cells/tissues
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Many basic functions of life utilise E eg
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Autotrophs
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Heterotrophs
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Three main classes of food nutrients
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Protiens (polypeptides) to aa
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Complex dietary polysaccharides such as starch are digested by
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Fats are emulsified in the
Important in fat oxidatioin (Fatty acid oxidation). Not 'essential' because body has some capacity to produce them from other compounds.
Acts as an antioxidant by protecting body against free radicals and oxidative stress
Imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects
Component of many redox enzymes including cytochrome c oxidase
-free radicals- can form as byproducts. These are oxidizers and some react very strongly with membranes and proteins causing cell/tissue damage.
Heart and nervous system because of their great need for energy.
It also destroyed vit C along with bacteria
L-ascorbic acid
May have desirable properties including antioxidant activity
Weight loss, emotional disturbances, impaired sensory perception, weakness and pain in limbs, irregular heartbeat and oedema. Heart failure. death.
Vitamins, minerals, herps or other botanicals, aa, enz, organ tissues, glandulars metabolites
Comination of nutrition and pharmaceutical: describes a product or purified form of foods that is sold in medicinal forms not usually associated with food and demonstrated to provide protection against or treatment of a disease
Eg plants can derive energy from sunlight
In trace amounts, catalytic role in enzymes/protein functions.
Omega-3 or omega-6
Peripheral neuropathy consisting of symmetric impairment of sensory, motor and reflex functions affecting distal more tha proximal limb segments => calf muscle tenderness
Eg mammals rely on degradation of chemical sources of fuel for energy
Thyroxine
The hame group of haemoglobin
Thiamine
Protect against chronic joint inflammatory diseases such as arthritis
Phytochemical that gives tomatoes their red colour, evidence of prevention of prostate cancer and atherosclerosis
Protiens (polypeptides) to aa - carbs (polysaccharides) to monosaccharides - fats to FA and monoacylglycerols
Tablets, capsules, softgels, gelcaps, liquids or powders
Pharmaceutical drugs
Wound-healing and preventing bleeding from capillaries
Chemical elements required by living organisms, many metals occur as ions in body
Water soluble vitamin of B complx. Its phosphate derivatives involved in celular processes (TPP - coenzyme in catabolism of carbs). Essential bitamin from diet.
In stomach acid denatures and partially digests with digestive enz
Growth, movement and metabolic processes like active transport or detoxification
Essential in high quantity; Ca, electrolyte and structurally for bone strength
damage to the body's peripheral nervous system. This can cause muscle weakness, numbness and tingling,
Be present in diet
Coenzyme for several enz that catlyze transfer of 2-C unit sin particuler it is coenz of PDH
Production of acetylcholine, neurotransmitter, and in myelin synthesis
One of principle classes of phytochemicals, chemicals known to destroy free radicals and reactive Oxygen species that cause CVD
Monounsaturated (1 C=C like oleic acid) or poly unsaturated (many C=C like eicosapentanoic acid)
Associated with mental confusion, muscular atrophy, edema, in addition to peripheral neuropathy
Essential nutrients in diet for good health. Vit D is exception; it can be synthesized in skin from cholesterol, in presence of UVB radiation from sunlight.
Eat fish oil to prevent inflammatory rxns of CVD and arthritis
Yellow pigmented carotenoid in many yellow/orange fruit/veg.
Vegetable oils in diet
Inflammatory hormones called prostaglandens
meats, tofu, eggs, legumes, milk cheese
Berberi: affecting peripheral nervous system (polyneuritis) and/ CVS with fatal possibilities
Antioxidant compounds for normal cell function, growth, and division
Dietary supplements, specific diets, GM foods, herbal products and processed foods such as cereals, soups and beverages.
Contains all eight chemically idstint B vitamins.these are a group of water-soluble vitamins that play important roles in cell metabolism.
Gluconeogenesis
Product containing nutrients derived from food products that are concentrated in liquid or capsule form
Omega 3 FA alpha-linoleic acid and omega-6 linoleic acid which gie rise to omega-3 eicosapentanoic acid (EPA and omega-6 arachidonic acid (AA)
C atoms double-bonded to H atoms
Collagen synthesis rxns
Standards function and effectiveness of neutraceuticals and dietary supplements
Digestion, absorption and assimilation
Essential and must be included in diet
Thighs and legs. Person looks pale and feels depressed, partially immobilized.
Synthesised collagen is too unstable to perform function without vit C
Fish oil
Triacylglycerol consists of 3 FA (long chains C-H atoms) bonded to glycerol
Amylase to release glucose
SI by bile acids to form small droplets (large SA). Lipases in intestine break down dietart TAG into FA and glycerol
All C atoms in their FA chains bonded to H atoms
Protein functions
Structural (muscles. skin)
Catalytic (enzymes)
Signalling (receptors)
Transport
Body requires AA to make new proteins
A-1,6 glucosidase
Hydrolysis a-1,6 linkage in glycogen => straight chain
Be used in TCA cycle in pyruvate form
Found on same bifunctional coenzyme as part of single 160,000 Da polypeptide chain
Be converted to glucose by glucose-6-phosphatase and released into bloodstream
Located on lumenal side of smooth ER to convert it to glucose
Phosphoglucomutase step as G-1-P>>>G-6-P
Glycogen breakdown transferase and phosphorylase activity
Hydrolysis a-1,6 linkage in glycogen => straight chain
Be used in TCA cycle in pyruvate form
Found on same bifunctional coenzyme as part of single 160,000 Da polypeptide chain
Be converted to glucose by glucose-6-phosphatase and released into bloodstream
Located on lumenal side of smooth ER to convert it to glucose
Phosphoglucomutase step as G-1-P>>>G-6-P
Glucose -1- phosphate is converted to Glucose - 6- phosphate due to reversal of
Hydrolysis a-1,6 linkage in glycogen => straight chain
Be used in TCA cycle in pyruvate form
Found on same bifunctional coenzyme as part of single 160,000 Da polypeptide chain
Be converted to glucose by glucose-6-phosphatase and released into bloodstream
Located on lumenal side of smooth ER to convert it to glucose
Phosphoglucomutase step as G-1-P>>>G-6-P
In skeletal muscle G-6-P will
Hydrolysis a-1,6 linkage in glycogen => straight chain
Be used in TCA cycle in pyruvate form
Found on same bifunctional coenzyme as part of single 160,000 Da polypeptide chain
Be converted to glucose by glucose-6-phosphatase and released into bloodstream
Located on lumenal side of smooth ER to convert it to glucose
Phosphoglucomutase step as G-1-P>>>G-6-P
In liver G-6-P will
Hydrolysis a-1,6 linkage in glycogen => straight chain
Be used in TCA cycle in pyruvate form
Found on same bifunctional coenzyme as part of single 160,000 Da polypeptide chain
Be converted to glucose by glucose-6-phosphatase and released into bloodstream
Located on lumenal side of smooth ER to convert it to glucose
Phosphoglucomutase step as G-1-P>>>G-6-P
G-6-P cannot diffuse out of cells therefore liver has enzyme
Hydrolysis a-1,6 linkage in glycogen => straight chain
Be used in TCA cycle in pyruvate form
Found on same bifunctional coenzyme as part of single 160,000 Da polypeptide chain
Be converted to glucose by glucose-6-phosphatase and released into bloodstream
Located on lumenal side of smooth ER to convert it to glucose
Phosphoglucomutase step as G-1-P>>>G-6-P
Phosphorolysis in that it is reversible in vitro (test tube) but in reality it is irreversible because
[Pi] is higher than [glucose 1 phosphate]
Active transport occurs using ATP to force reaction forward
More energetically favourable; formation of phosphorylated glucose with using energy ATP.
More energetically favourable; formation of phosphorylated glucose w/o using energy - ATP.
Phosphate group is energetically stabilised by resonance, but not the case when in ATP
[glucose 1 phosphate] is higher than [Pi]
Anabolic rxns
Catabolic rxns
Rxns not requiring oxygen (anaerobic)
Rxns requiring oxygen (aerobic)
Isoelectric point
Ph where aa no net charge
PI = pK1+pk2
Ph where no charges exist
PI = (pk1+pk2)/2
Optical isomerism of the a C of aa found in proteins always
D
Relates to D-glyceraldehyde
R
S
Rotates plane polarized light to right
Relates to L-glyceraldehyde
Rotates plane polarized light to left
L
In biochem use system of relative configuration because
It gives the shape of the molecule
All molecules designated L relate to L-glyceraldehyde
It gives absolute configuration
precise arrangement of substituents at a stereogenic center
Arrangement of atoms in an optically active molecule, based on chemical interconversion from or to a known compound
There is no way of knowing just by looking at a structure whether the assignment of (+) or (-) is correlated to a particular isomer, R or S
Protein aa side chains differ properties in terms of
Size
Shape
Charge
Colour
H bonding
Length
Capacity
Chemical reactivity
Genetic code
Substrates
Imino
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Sulphur
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Aliphatic
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Hydroxyl
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Amide
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Acidic
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Aromatic
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Basic
phenylalanine (phe), tyrosine (tyr), tryptophan (trp)
Asparagine (asn), glutamine (gln)
lysine (lys), arginine (arg), histidine (his)
Serine (ser), threonine (thr)
glycine (gly), alanine (ala), valine (val), leucine (leu), isoleucine (ile).
Proline (pro)
aspartic acid (asp), glutamic acid(glu)
Cysteine (cys), methionine (met)
Plasma membrane is responsible for
maintaining the internal environment (pH, osmolality, ionic - Na+, K+, Ca2+, Cl- etc.) so that the delicate biochemical machinery can function properly.
control the intake of food and fuel, and to get rid of waste products.
Lipid bilayer readily permeable only to
Lipid soluble mol
O, N, CO2, S, H, NO2
Small uncharged molecules such as urea, glycerol
Water
O, N, CO2
Other water soluble mol/ions selectively transported via channels/pumps/gates formed by transmembrane proteins. In general transport is of two types.
Passive diffusion
Active transport
Co transport systems
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Passive diffusion
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
UNIPORT
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Uptake of sugars/aa in animal cell slinked to inward Na movement. Na/K ATPase sodium pump is
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Active transport
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Many active transport systems are driven by the ______ rather than directly by the use of ATP
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Sodium symport
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
In glucose transport, glucose is released into circulation on other side of epithelium cell by
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Sodium pump
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
The active transport of Na+ out of the cell by the sodium pump creates a gradient which links to
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
The overall effect is that glucose is transported from
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Glucose moves into the cell with the sodium ions and is then
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
exist also in internal membranes of cell. Provide means = organelles (mitochondria, ER) can maintain an internal ionic environment diff.from that of cytoplasm.
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Example ion pump
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
The concentration of H+ in the mitochondrial matrix is linked to
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Ionic gradient in ETC/phosphorylation used to
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Some ion channels are not permanently open but open
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Ligand gated ion channel
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
Voltage gated ion channel
When a transport protein simply transports one solute from one side of the membrane to the other
phosphorylation of ADP to produce ATP.
Energy stored in ion gradients
Maintians gradient for sodium symport
Antiport - transporting K+ and Na+ against conc gradients at expense of hydrolysis of ATP
The intestine, across the epithelial cell and into the inter cellular space, then into the blood.
voltage sensitive sodium channel of nerves.
transport of ions or solutes across the membrane against their concentration gradients and REQUIRES METABOLIC ENERGY (ATP) DIRECTLY OR INDIRECTLY.
nicotinic acetylcholine receptor.
Transported out of the cell at the other end down its concentration gradient via a carrier protein
simple (across membrane unaided or via channel) or facilitated (using specific carrier proteins) - solute or ion is conveyed down its concentration gradient WITHOUT THE EXPENDITURE OF METABOLIC ENERGY.
The uptake of nutrients such as glucose and amino acids into epithelial cells
ER: Ca2+ -ATPase which pumps Ca2+ out of the cytoplasm to keep its concentration very low and to build up a store of Ca2+ in the E.R. Which can be released when required
facilitated diffusion down its concentration gradient.
Make ATP as the end product of catabolism; in contrast to the sodium pump which uses ATP to create an ionic gradient across the plasma membrane.
transiently only in response to a particular stimulus. These GATED ion channels are of two types
Ion pumps
the inward flow of Na+ at the other end of the cell by the Na+ / glucose symport.
Of one solute depends on the simultaneous or sequential transfer of a second solute, either in the same direction (symport) or in the opposite direction (antiport).
cells also take in bulk quantities of the extracellular fluid, macromolecules and large particles such as other cells by the separate process of
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
. The uptake of fluid alone is often termed
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
The engulfing of large particles or cells is known as
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Specialised cells for phagocytosis; engulf dead cells/debris first line defence against invading microorganisms
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Process of endocytosis possible because
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
It can flow around a large particle or a volume of medium and fuse to form an endocytic vesicle which is taken into the cell
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
. In some cases endocytic vesicles fuse with lysosomes and their contents are broken down by
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Certain molecules can be selectively taken up by binding to a specific receptor protein at the cell surface which
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Cholesterol mol example of
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Synthesised in the liver and transported in the bloodstream in the form of low-density
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Cholesterol needed by cells
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Enzymes in the ______release the cholesterol for the cell’s use.
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Receptor-mediated endocytosis confined to areas called
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
At sites where receptor mediated endocytosis takes place
LDL receptors bind the clathrin which keeps them localized in one area.
Endocytosis membrane
Macrophages
Cholesterol
lysosomal enzymes.
Pinocytosis; occurs routinely in virutally al animal cells
Then triggers internalisation of part of membrane to form endocytic vesicle
Receptor-mediated endocytosis
for their membranes and have special LDL receptors on their surface which bind LDL and the whole complex is internalized.
Membrane is fluid
endocytic vesicle
Clathrin-coated pits
Endocytosis
Phagocytosis
Most cells make and release material to the exterior
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Examples of secretion
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Few can pass through the membrane but have to be released by
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Material to be released is enclosed in small membranous vesicles which fuse with the
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
E vesicles are variously described as
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Proteins are packaged in the
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Small molecules such as neurotransmitters are manufactured
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Secretion can be
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Common features of Biological Membranes ; Consist of lipids and proteins mainly;
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Common features of Biological Membranes ;. Lipids are amphipathic -
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
A common factor triggering exocytosis in many cells in response to a variety of stimuli is a
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Common features of Biological Membranes 1. Sheetlike structure only
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Common features of Biological Membranes ; Specific proteins mediate specific functions
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Common features of Biological Membranes ;
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Common features of Biological Membranes ; biological membranes are
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Common features of Biological Membranes ; membranes are fluid
Enzymes, peptide and steroid hormones, materials to form the extracellular matrix
pumps, gates, channels, receptors, enzymes
secretory vesicles, zymogen granules or synaptic vesicles.
Golgi apparatus
Plasma membrane delivering their contents to the exterior.
co-operative NONCOVALENT assemblies.
Lipid molecules diffuse in plane as do proteins, unless anchored.
In cytoplasm and actively transported into preformed vesicles
spontaneously form lipid bilayers. Polar molecules cannot cross the lipid bilayer.
sudden rise in the concentration of intracellular free calcium ions.
a few molecules thick 60 - 100Å across, form closed boundaries.
Asymmetric - inside and outside faces different.
Exocytosis
Some carbohydrate, proportions vary between different membranes.
Continuous or as the result of the cell being stimulated by an appropriate signal, such as a hormone or nerve impulse.
Secretion
Membrane transport 1. Lipid-soluble molecules pass through the lipid bilayer.
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
EXOCYTOSIS
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
ENDOCYTOSIS
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
Because the mebrane is fluid it can pinch off to form vesicles to transport material by
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
Large particles can be engulfed by the process of
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
bulk transport of volumes of external medium are encapsulated by pieces of membrane and taken in to the cell by
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
Channel proteins can be
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
Transport can be classified as: PASSIVE I.e. Down the concentration gradient.
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
ACTIVE TRANSPORT
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
water may be transported by special mechanisms using channels called
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
Water-soluble molecules and ions have
ENDOCYTOSIS
May be adapted for specific transport by receptor-mediated endocytosis.
PHAGOCYTOSIS
Specialised transport systems
PINOCYTOSIS
Also oxygen and carbon dioxide, water and small uncharged molecules. 2
. This may be simple diffusion (unaided or through an open channel protein) facilitated diffusion (using a specific carrier proteins)
movement of ions or solutes across the membrane against their concentration gradients using specific proteins; requires energy.
AQUAPORINS
Cells secrete molecules to the exterior by vesicle formation and fusion with the plasma membrane -
ligand or voltage gated.
SNARE proteins
Synaptobrevin
Synaptovin
Synapse
SNAP-5
SNAP-25
Syntaxin
Synaptotagmine
Synaptodrenin
Synaptotagmin
Synaxin
Synaptotagmin
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
NMJ presynaptic neurone
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Post synaptic neuron NMJ
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Eukaryotic membranes contain ___ which cause disruption of the ordered bilayer structure
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Membrane: diff phospholipids, unsaturated FA + cholestorl means biol. mem <=> synthetic mem, are
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Lipids often found to be arranged
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Acetylcholinesterase
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Human RBC, sphingomyelin and phosphatidylcholine located on
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Liposomes
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Deliver cargo directly into cell by coalescing with cell mem
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Human RBC. Located on external surface
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Human RBC exclusively forming inner layer
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Although it is possible for lipids to diffuse sideways within a bilayer,
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Proposed in the early 1970’s by Singer & Nicholson
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Fluid mosaic model
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Choline acetyltransferase
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Acetate goes via blood and excreted. Other byproduct choline from ACh breakdown
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Excitation-contraction coupling
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Flexible assembly held by noncovalent interactions, lipid-lipid/protein-lipid. Both can move
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Ut some proteins are relatively immobile, and are believed to be
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
The proteins in the membrane (sometimes described as floating like icebergs in a sea of lipid) function as
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Once proteins are inserted in the membranes they
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Peripheral proteins
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
AC in skeletal muscle plasma membrane propogates down muscle fibre into
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Integral proteins
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Many membrane proteins are GLYCOPROTEINS having groups such as
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
T tubules have voltage sensors
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Voltage sensors linked anatomically and functionally to
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Endplate AC => voltage sensors => Ca2+ released from
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
The proteins in a cell membrane can be solubilised, separated and visualised using
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Since not possible to obtain 3D structure of proteins by X-ray crystallography,
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Amino acid sequence of a membrane protein (determined directly or from the nucleotide sequence of its gene) can be interpreted
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Example of hydrophilic carb side chain
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
hydrophilic carbohydrate side chains invariably are found on
Small hollow spheres of lipid membranes for non soluble material/drug delivery. Phospholipids in aq environ.
High proportion of sterols
Liposomes
Sideways in plane of membrane
Asymmetrically in cell membrane
Fluid mosaic model
Glycoproteins including histocompatbility antigens by which cell recognised.
Cleaves acetylcholine at post synaptic membrane into choline and acetate
Anchored to the cell’s cytoskeleton
loosely attached and easily removed by mild treatment, such as altering pH or ionic strength
Firmly embedded, only removed by disrupting the membrane with detergents or organic solvents
N-acetylglucosamine and N-acetylgalactosamine attached to serine or asparagine residues
To make ACh from choline and acetyl coA
Lipid bilayer in which proteins float, some spannign membrane (transmembrane), some attached only to interior/exterior (peripheral proteins)
Binds Ca2+ to change arrangement proteins so viseicle fuses with presynaptic membrane => pore => exocytosis
Returns to presynaptic terminal and recycled
Process by which neurotransmission at NMJ causes skeletal muscle contractions
S gates, pumps, channels, receptors, coupling proteins, enzymes etc
Always fluid at physiological temperatures
T tubules (transverse) which penetrate deep into muscle fibres (myofibrils)
Don't change their orientation
Sphingomyelin and phosphatidylcholine
Ligand gated ion channels (nicotinic rec)
Phosphatidyl ehtanolamine and phosphatidylserine
, there is a large energy barrier to any movement between the two layers (`flip-flop’).
Sacroplasmic reticulum via ryanodine receptors (ligand gated ion channel stimulus for contraction) into cytoplasm
Sacroplasmic reticulum
External surfaces
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
Shape of an integral protein in a membrane is found mostly from aa sequence and electron micrographs.
Hydrophobic regions anchored in hydrophobic environ. And hydrophilic regions of aa charged/polar side groups protrude from either cytoplasmic or external mem face.
Voltage gated ion channels
portion of the protein protruding from the outer face of the membrane
Dihydropyridine receptors
Neuromuscular blockers do not
Block pain
Cause skeletal muscle paralysis
Consist of depolarising and non-depolarising substances
Depolarising neuromuscular blocker
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Phospholipids and cholesterol
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Cholesterol and FA
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
TAG
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Cholesterol and FA
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
FA
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Fatty acids have both hydrophobic and hydrophilic components, and their properties depend on:
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Saturated FA more than 8 Carbons are
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Oleic acid
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Naturally occurring saturated fatty acids that have from 1 to 8 carbons are
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Increase in C=C in H-C chains
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
When a double bond is found in the H-C chain of a FA
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Most unsaturated FA are found as the
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Suxemethonium is agonist initially activating skeletal muscle
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Suxemethonium used to paralyse
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Non depolarising blockers are used as adjunct to general anaesthetics to cause
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Competitive reversible antagonists at nicotinic receptors.
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
FA with 2 or 3 C=C are
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Presence of C=C in FA shape
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Non depolarising blockers
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
. Fatty acids with two or three double bonds (linolenic acids has three double bonds) are known as
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Acetylcholinesterase blockers
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
polyunsaturated fatty acids are the precursors of some very important signal molecules
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
The balance of w3 and w6 in the diet is extremely important.
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
TAG that are solid at room temperature
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
�oils” (e.g. olive, corn and peanut oils).
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Edrophonium
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Carbamates quasi irreversible inhibitors with 1-3 h duration action
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Organophosphates
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Dyflos Malathion and Parathion
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Condensation of each alcohol (-OH) of glycerol with a carboxyl group (-COOH) of a fatty acid forms an
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Three molecules of fatty acid (of the same or different kinds) condense
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Phospholipids most important structurla lipids
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Phospholipids are ampipathic
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
complex 27C lipid with 4 rings and an aliphatic chain.
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Obtained from the diet and synthesized
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Cholesterol important structural role in membranes and is a precursor of steroid hormone,
Polyunsaturated FA omega 3 (fish oils) and omega 6(plant oils)
SOLID
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
With glycerol, with the removal of water.
Blocks enz => increase in ACh lfietime in synaptic cleft - Physostigmine, Pyrostigmine, Neostigmine, and Edrophonium
The length of the hydrocarbon chain, • double bonds (if present)
hydrophilic polar head groups (phosphate group and nitrogenous group) facing aqueous areas and hydrophobic fatty acid tails buried in the membrane’s nonpolar interior
C18 cis9,10
Not occupy min space as straight chains. large, bulky, bent, important for fluidity
Competitive reversible inhibitor of ACh with short 15 min duration
Nicotinic receptors but causes rapid receptor desensitisation hence paralysis
Ester linkage
ESSENTIAL FATTY ACIDS because we cannot synthesize them
Bind irreversibly to acetylcholinesterase
Fats
Similar to TAG, but 1 FA replaced by P gropu usually linked to small N containing croup eg. choline, serine, ethanolamine
mainly in liver from carbohydrate precursors
Vitamin D and bile acid synthesis
Liquid
Eg pancuronium vecuronium and atracurium
Larynx to allow tracheal intubation
Lowers melting point
Eg arachidonic acid.
less stable cis configuration, rather than the more stable trans form.
Liquid at RTP, and usually have more unsaturated FA. More common in plants than animals
Geometric isomerism occurs
Physostigmine, pyridostigmine, neostigmine
Organophosphates largely used as pesticides tho malathion also active ingredient for head lice treatment
Precursors of important signalling molecules (Steroid hormones and prostaglandins)
Non depolarising blockers
Rincipal component of lipids. unbranched H-C chain with Carboxyl at one end, aliphatic chain of C. Saturated or have 1-6 C=C. Chemical structure <= physical properties <= basis of biol. function
Widespread skeletal muscle paralysis during major surgical procedures
Cholesterol
Components of membranes outer boundary of eukaryotes and separate aq compartments from each other in cells/organelles
Unsaturated fatty acids
Suxamethonium (used anymore?)
Destruction of an enzyme activity by protein denaturation e.g., strong acids or bases etc.
Is not regarded as enz inhibition
Is reversible
Is irreversible
Is regarded as enz inhibition
Irreversible inhibitors
E + I <=> EI
Progressive effect reaching max when all enz reacted
Not easily reversed by simple physical treatments
Same as quasi-irreversible inhibitors
Instantaneous effect
Bind very tightly to enz sometimes by covalent bonds making E-I compound
More potent than reversible
Can be removed from enz by dialysis or dilution, returning activity to normal
Interact with enzyme by weak noncovalent bonds forming EI complex
E + I => EI
For a given enzyme, Km is a constant and is thus
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
The simplest possible scheme for an enzyme reaction I
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
After extraction of an enzyme from a tissue, its reaction is studied
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
The physical conditions under which the assay is performed will determine
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
The velocity of an enzyme reaction is
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Molecules which closely resemble the substrate in size, shape and charge distribution may also fit into the active site of the enzyme
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Orbital steering
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Enzymes may provide functional groups capable of
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Binding substrates in the active site may bring them into
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Some enzymes combine with the substrate to form an unstable covalent intermediate in a
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Binding substrates in the active site increases the
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
The functional groups on side chains of aa interact with substrate through
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
The amino acids forming the active site are few and are far apart in the sequence but
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
The enzyme may induce strain or distortion in the
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
AChE Anionic subsit
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
General acid - base catalysis
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Why tryptophan 84 essential in anionic subsite of AChE?
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Anionic subsite aa
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
In membranes, Main class of lipids are phospholipids consisting of FA linked to glycerol OR sphingosine which are linked via
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Esteratic subsite is where acetylcholine is
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Esteratic subsite; A catalytic triad of three amino acid sidechains: serine 203, histidine 447, and glutamic acid 334
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
ENZYME ASSAY.
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
It is important to measure the velocity before any of these factors can affect the reaction I.e. Always measure
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Impurities in the enzyme and product become more
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
The amount of enzyme present in a tissue sample is taken from an assay of activity assuming that this linear relationship holds.
Close proximity to a catalytic group
the INITIAL VELOCITY, when the reaction is linear, otherwise the measurements may be misleading.
It just is.
Hydrolysed to choline and acetate.
weak noncovalent forces such as ionic bonds, hydrogen bonds and hydrophobic bonds and position the substrate within the active site.
independent of enzyme concentration.
E binds the positive quaternary amine of the acetylcholine.
Effective concentration of reactants
in vitro by mixing enzyme extract and substrate together under suitable conditions
important as the concentration increases
A “transition state” that more readily undergoes reaction to form products. Substrates in the active site are so oriented that a transition state is readily formed.
enzyme may position substrates in its active site and lines up molecules very precisely for reaction so that bonds can be broken or made more easily
Spatially close due to 3D structure
Susceptible bond, conformational change part of induced fit theory
found in the reactions of the dehydrogenases, the serine proteases, thiol proteases and carboxypeptidase, ribonuclease and lysozyme.
Acting as proton donors/acceptors; general acid base catalysis
Phosphate group to headgroup eg Ser, Choline, Ethanolamine or sugar alcohol inositol.
Substrate converted into product by enzyme action.
Important in clinical measurements
4 attack the ester bond through the formation of a transition state in which the substrate is covalently bound to the serine prior to hydrolysis of the ester bond.
Disappearance sub,appearanc product, measured by appropriate analytical technique such as colorimetry
Fourteen aromatic aa line the gorge leading to the active site
Competitive inhibition
Enzyme activity. Determined by factors which need to be studied in turn while others remain constant
Change in reactant or product with time
Nomenclature; Oxidoreductases (or Dehydrogenases)
� transfer functional groups from donor to acceptor
. Pyruvate decarboxylase – break bonds such as C-C, C-O, C-N..
E.g. Alanine racemase – geometric or structural changes within a molecule
E.g. Glutamine synthetase – joins molecules together, forms bonds, requiring ATP energy
Enzyme Commission Number
E.g lactate dehydrogenase – catalyse oxidation and reduction reactions.
S e.g. esterases (acetylcholinesterase), proteases – hydrolysis of C-O, C-N and C-C bonds
Transferases e.g. Aspartate aminotransferase
� transfer functional groups from donor to acceptor
. Pyruvate decarboxylase – break bonds such as C-C, C-O, C-N..
E.g. Alanine racemase – geometric or structural changes within a molecule
E.g. Glutamine synthetase – joins molecules together, forms bonds, requiring ATP energy
Enzyme Commission Number
E.g lactate dehydrogenase – catalyse oxidation and reduction reactions.
S e.g. esterases (acetylcholinesterase), proteases – hydrolysis of C-O, C-N and C-C bonds
Hydrolases e.g. esterases (acetylcholinesterase), proteases
� transfer functional groups from donor to acceptor
. Pyruvate decarboxylase – break bonds such as C-C, C-O, C-N..
E.g. Alanine racemase – geometric or structural changes within a molecule
E.g. Glutamine synthetase – joins molecules together, forms bonds, requiring ATP energy
Enzyme Commission Number
E.g lactate dehydrogenase – catalyse oxidation and reduction reactions.
S e.g. esterases (acetylcholinesterase), proteases – hydrolysis of C-O, C-N and C-C bonds
Lyases
� transfer functional groups from donor to acceptor
. Pyruvate decarboxylase – break bonds such as C-C, C-O, C-N..
E.g. Alanine racemase – geometric or structural changes within a molecule
E.g. Glutamine synthetase – joins molecules together, forms bonds, requiring ATP energy
Enzyme Commission Number
E.g lactate dehydrogenase – catalyse oxidation and reduction reactions.
S e.g. esterases (acetylcholinesterase), proteases – hydrolysis of C-O, C-N and C-C bonds
Isomerases
� transfer functional groups from donor to acceptor
. Pyruvate decarboxylase – break bonds such as C-C, C-O, C-N..
E.g. Alanine racemase – geometric or structural changes within a molecule
E.g. Glutamine synthetase – joins molecules together, forms bonds, requiring ATP energy
Enzyme Commission Number
E.g lactate dehydrogenase – catalyse oxidation and reduction reactions.
S e.g. esterases (acetylcholinesterase), proteases – hydrolysis of C-O, C-N and C-C bonds
Ligases
� transfer functional groups from donor to acceptor
. Pyruvate decarboxylase – break bonds such as C-C, C-O, C-N..
E.g. Alanine racemase – geometric or structural changes within a molecule
E.g. Glutamine synthetase – joins molecules together, forms bonds, requiring ATP energy
Enzyme Commission Number
E.g lactate dehydrogenase – catalyse oxidation and reduction reactions.
S e.g. esterases (acetylcholinesterase), proteases – hydrolysis of C-O, C-N and C-C bonds
Subdivisions in each group according to the exact substrate allow a complete classification, each enzyme with its own
� transfer functional groups from donor to acceptor
. Pyruvate decarboxylase – break bonds such as C-C, C-O, C-N..
E.g. Alanine racemase – geometric or structural changes within a molecule
E.g. Glutamine synthetase – joins molecules together, forms bonds, requiring ATP energy
Enzyme Commission Number
E.g lactate dehydrogenase – catalyse oxidation and reduction reactions.
S e.g. esterases (acetylcholinesterase), proteases – hydrolysis of C-O, C-N and C-C bonds
Since enzymes are proteins containing many ionisable groups, they exist in a whole series of different states of ionisation
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
PH
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
Additional substance besides the enzyme and substrate is required in order that the reaction may proceed.
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
Coenzymes are
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
Cofactors
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
Coenzymes (or co-substrates) are
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
Prosthetic groups are present in the same
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
Simple inorganic ions are often needed in enzyme catalysis e.g.
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
S initial velocity tends towards a maximum - Vmax I.e.
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
When initial velocity is plotted against substrate concentration,
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
The value of Vmax will vary with the concentration of
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
plot does not plateau out abruptly
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
NOT possible to calculate a true value for Vmax from this plot because
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
The inhibitor has a separate equilibrium
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
The Michaelis Constant (Km)
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
To obtain accurate values for Km and Vmax, the experimental results for the variation of initial velocity with substrate concentration are plotted not as the hyperbola,
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
Lineweaver burke plot
is the concentration of substrate that gives half maximal activity (1/2 Vmax) and has the dimensions of concentration I.e. Mol l-1 .
Approaches vmax hyperbolically
When all available enzyme is saturated with substrate.
Enz used
Concentration as the enzyme (and not easily removed).
Which depend on pH
Y took reciprocals of the Michaelis - Menten equation and plotted 1/v against 1/[S]
Stoichiometric with the substrate (and easily removed)
the graph obtained is a rectangular hyperbola.
S take part in the chemical reaction catalysed by the enzyme, often as carriers of a particular group.
With the enzyme
Vmax ony achieved at infinite substrate concentration
complex organic molecules derived from the water soluble vitamins.
Organic coenzymes or prosthetic groups. • Inorganic ions or activators.
But are subjected to a linearising transformation. The most famous of these transformations is that due to Lineweaver and Burk
Mg2+, Mn2+, Zn2+, Cu2+ etc.
greatest effect in the active site where ionization may affect shape and any ionic binding of substrate
For a given enzyme, Km is a constant and is thus
Independent of enzyme conc
Dependent of enz conc
Independent of subs conc
Dependent on subs conc
Vmax is proportional to enzyme concentration
actual enzyme concentration [E] is known
turnover number are time -1 , I.e. sec-1 or min -1
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
why do we want to know Km and Vmax? –
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Km is a dissociation constant, and 1/Km is a
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Vmax is a
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
The turnover number gives
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
enzymes that comprise one polypeptide chain folded to produce
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Some enzymes are more complex and have quaternary structure with
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Because I and S compete for the same site, if [S] is increased sufficiently while [I] is constant
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
. If sufficient [S] is present then eventually
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
The concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor.
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
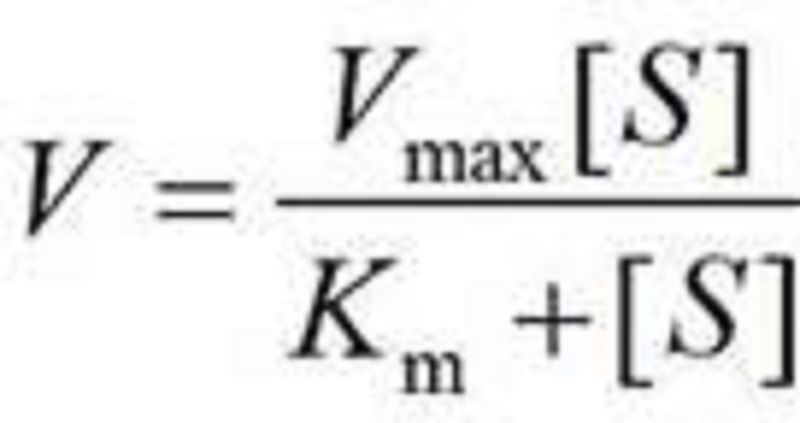
What eq is this, make sure you know corresponding graph
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
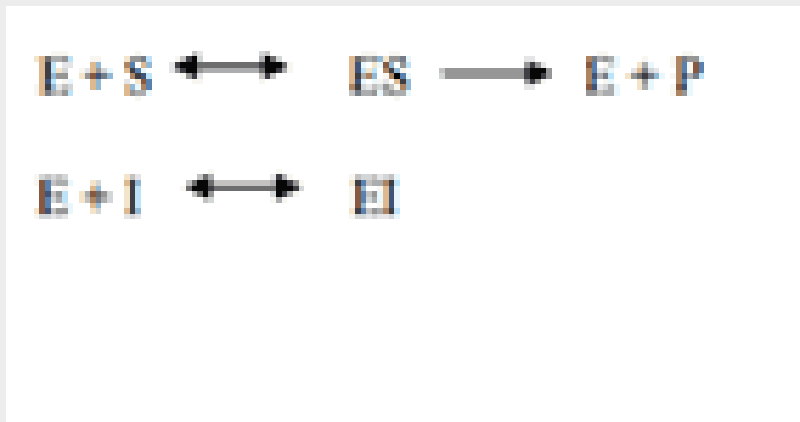
Each of these equilibria is characterised by a dissociation constant.
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Competitive reversible inhibition; The binding of substrate and inhibitor is
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
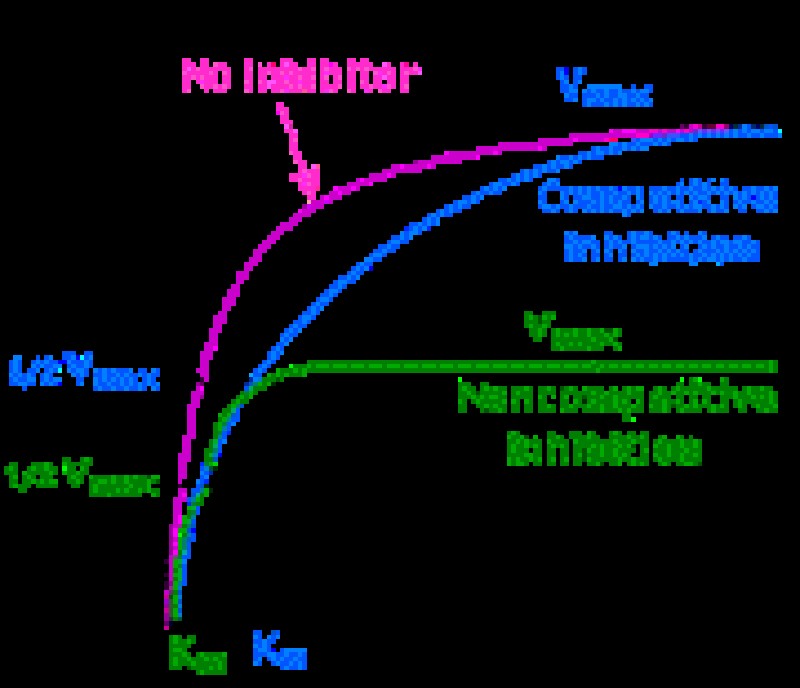
What eq is this, make sure you know corresponding equation
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
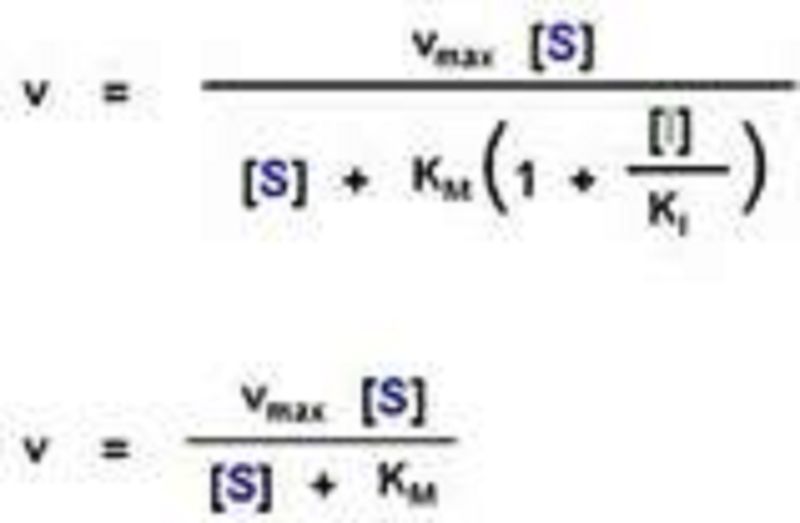
What eq is this make sure you know corresponding graph
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
The most accurate way of determining Ki is from a
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
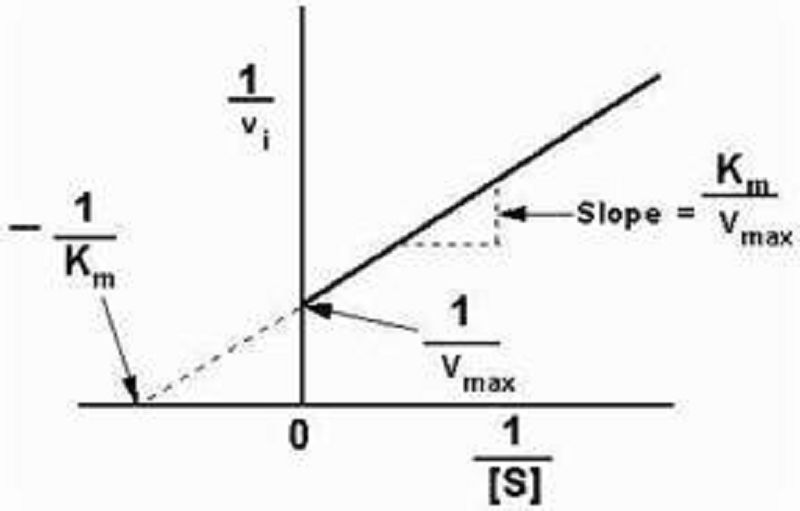
What eq is this and make sure you know equation.
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Lineweaver burke plot
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
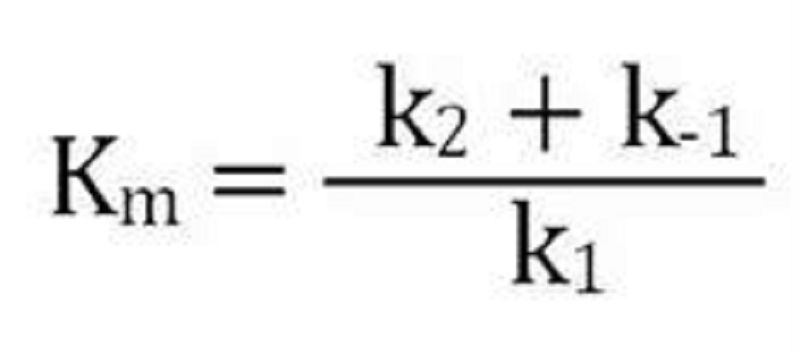
What eq is this
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Michaelis constant
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Ki is the
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
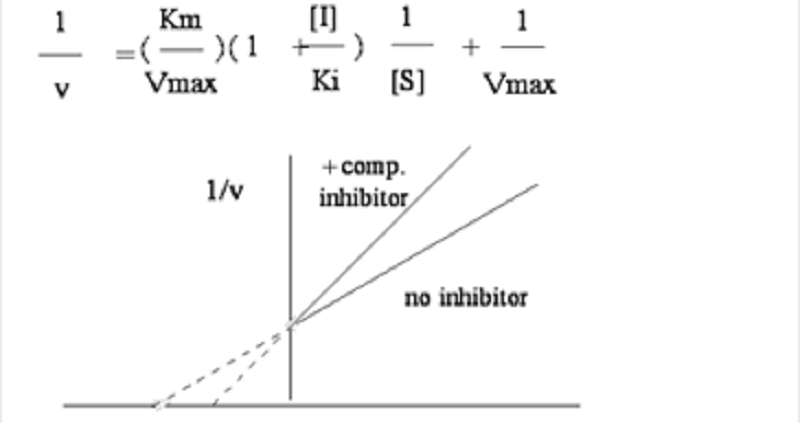
The intercept on y axis represents 1/Vmax (when 1/[S] = 0, [S] is infinite). Same with/without inhibitor. This is an important diagnostic test for this type of inhibitor. The slope is altered by the factor 1 + [I]/Ki, but the
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
In competitive reversible inhibition the vmax
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Without inhibitor the intercept is -1//Km, with inhibitor it is -1/Km(1+[I]/Ki), so the ratio (bigger over smaller so it is greater than 1) is
one active site where one substrate binds
The most accurate way of determining Ki
multiple subunits or multiple active sites within one single protein chain.
Concentration of inhibitor required to slow the reaction to half the rate it shows in the absence of inhibitor. It is a reciprocal measure of the affinity of E and I. / dissociation constant for the equilibrium between E and I
Mutually exclusive
the proportion of E attracted to I will decrease, hence formation of ES will increase and the rate of enzyme action will increase.
Line-weaver burke plot
First by Km (the Michaelis constant) and the second by Ki which characterises the binding between enzyme and inhibitor.
Ki
Easiest way to calculate Ki is from the ratio of the intercepts on the x axis.
1 + [I]/Ki.
quantitative assessment of the extent of catalysis.
Michaelis menton graph
Michael menton with competitive inhibition
Michaelis constant
Lineweaver-Burk plot which is derived by taking reciprocals of the above equations.
The inhibition by I will be overcome
Michaelis menton equation
Reciprocal measure of affinity of E and S
to characterize an enzyme and be able to measure quantitative effects of drugs/inhibitors on its action.
the rate constant for the catalytic reaction. Enzyme turnover numbers range from 10 - 107 per sec
Remains unchanged
This figure gives a good measure of the catalytic power of the enzyme
Measure of the affinity between enzyme and substrate. We can tell how well a substrate binds to its enzyme
Calculating Michaelis - Menten enzyme kinetics we make assumptions:
Enzyme - Substrate complex is formed (Xray crystallography)
Km is always half of Vmax, measurement of affinity between enzyme and substrate. How good binding is.
Turnover rate is constant for every catalytic reaction
the equilibrium where the rate of ES formation is equal to its breakdown is reached instantaneously.
Equilibrium rate constant is achieved and shown by initial velocity on lineweaver burke plot
There is no k-2 I.e. There is no chance of E + P forming ES – it is an irreversible reaction.
There is reversible reaction of ES => E+S and E+S => ES
[substrate] in ES is negligible; [Enzyme] probably 0.001% of the substrate concentration, so the proportion of substrate locked up in the ES complex is very small.
[enzyme] in ES is neglibible; [Substrate] probably 0.001% of the enzyme concentration, so the proportion of enzyme in the ES complex is very small.
Allosteric Enz; cooperativity
show the simple hyperbolic Michaelis - Menten type kinetic pattern
Initial binding of substrate to one subunit rapidly deccelerates binding to other subunits as substrate concentration increases.
Allosteric regulatory site distinct from the catalytic active site
they produce a sigmoidal pattern on a LINEAR initial velocity vs. Substrate concentration graph.
Allosteric regulatory site equivalent to the catalytic active site
In these enzymes, binding of substrate to one subunit will increase the affinity of other subunits for the substrate
binding of an activator or inhibitor molecule (effector) which is structurally related to the substrate
stimulate or inhibit the enzyme activity by changing the shape of the active site and thus changing its affinity for substrate.
effector could be
Another molecule in the metabolic pathway
Vmax value
A km measurement
Substrate concentration
Structurally related to substrate
Binding to active site
Stimulatory or inhibitory / activator or inhibitor
an ion such as Ca2+
In some cases, the regulatory and catalytic sites are on different types of subunit
e.g Acetylcholinesterase
e.g lysozyme
e.g aspartate transcarbamylase
This enzyme has six catalytic subunits and six regulatory subunits.
This enzyme has three catalytic subunits and regulatory subunits.
Anionic subunit and esteratic subunit
One hydrolyses and another reduces
Called multienzyme
An enzyme with multiple molecular forms (in the same organism) but catalysing the same reaction is known as
Isozyme
Lysozyme
Opticla isomer
Hydrolase
Dehydrogenase
lactic dehydrogenase (LDH) that occurs in FIVE possible forms in mammals
Isozyme
7 catalytic activities in single polypeptide chain
Multifunctional enzyme
Tetramer made of two types of subunits M and H
Similar to FAS
Different kinetic properties depending on physiological role
Same molecular weignt but different aa compositions. Products of two separate genes
Multienzyme complex
Similar to PDH
a-ketoglutarate dehydrogenase
Multienzyme complex
Same as multifunctional enzyme
a-ketoglutarate dehydrogenase
5 possible forms in mammals
Lactic dehydrogenase
Advantages in terms of speed, control and absence of interference
3 types of subunit
PDH
7 different catalytic activities in 1 polypeptide
association of enzymes (i.e. Different polypeptides) together, several enzymes are organised physically so that the product of one becomes the substrate of another
Effective assembly series of enz; highly efficient progression from reactants to products
FAS
Such complexes protect intermediates from competing rxns taking place in metabolism; would channel them away from making the desired product.
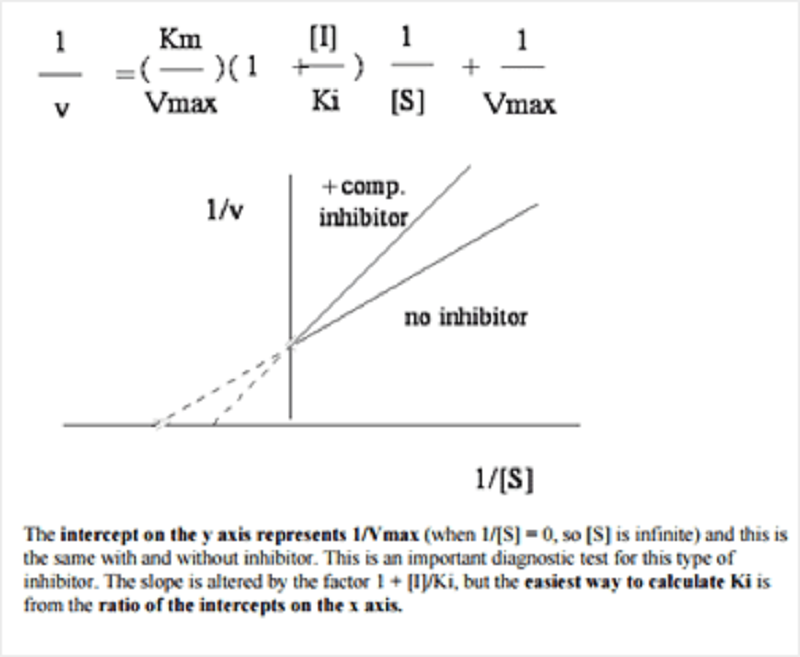
Without inhibitor the intercept is -1//Km, with inhibitor it is -1/Km(1+[I]/Ki), so
The ratio (bigger over smaller so it is less than 1) is 1 + [I]/Ki.
The ratio (bigger over smaller so it is greater than 1) is 1 + [I]/Ki.
The ratio (smaller over bigger so it is greater than 1) is 1 + [I]/Ki.
The ratio (smaller over bigger so it is less than 1) is 1 + [I]/Ki.
The ratio (bigger over smaller so it is greater than 1) is 1 - [I]/Ki.
The ratio (bigger over smaller so it is less than 1) is 1 - [I]/Ki.
The ratio (smaller over bigger so it is greater than 1) is 1 - [I]/Ki.
The ratio (smaller over bigger so it is lesser than 1) is 1 - [I]/Ki.
Irreversible enz inhibition
To work in vivo as an enzyme inhibitor
compound is a nonspecific enzyme inhibitor
Enz activity is inhibited for times significantly longer than the assay times for the enz.
Turnover of enz protein in vivo
Turnover of enz protein in vitro
inhibition is reversible in theory but irreversible in practice.
tight-binding inhibitors, transition state analogues and slowly dissociating intermediates.
Timescale of inhibition similar to that of enz action, measured over few mins
Tight-Binding inhibitors and Transition State Analogues form
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Tight-Binding inhibitors and Transition State Analogues form
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Slowly Dissociating Intermediates react with the enzyme to form
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Slowly Dissociating Intermediates react with the enzyme to form
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Quasi irreversible inhibitors
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Quasi irreversible inhibitors
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Slowly Dissociating Intermediates react with the enzyme to form
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Slowly Dissociating Intermediates react with the enzyme to form
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Compounds which fall into the irreversible category may be
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Compounds which fall into the irreversible category may be
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
For a compound to work as a drug in vivo it will ideally have
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
For a compound to work as a drug in vivo it will ideally have
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Many poisons work because they are relatively
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Many poisons work because they are relatively
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Potency
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Potency
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Specificity
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
Specificity
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
If an enzyme is not rate limiting, it may be necessary to achieve
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
If an enzyme is not rate limiting, it may be necessary to achieve
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
simple reversible inhibitors are unlikely to be
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
simple reversible inhibitors are unlikely to be
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
To work in vivo inhibitor needs to be
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
To work in vivo inhibitor needs to be
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
If a compound is a nonspecific enzyme inhibitor it is
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
If a compound is a nonspecific enzyme inhibitor it is
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
If an enzyme is not rate limiting, it may be necessary to achieve >90% inhibition before any increase in substrate concentration occurs.
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
If an enzyme is not rate limiting, it may be necessary to achieve >90% inhibition before any increase in substrate concentration occurs.
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
It is possible that a reversible competitive inhibitor which is a Conformationally Restricted Competitive Inhibitor will have a much
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
It is possible that a reversible competitive inhibitor which is a Conformationally Restricted Competitive Inhibitor will have a much
Nonspecific of certain types of enzymes
Potency and specificity:
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme.
Tight binding inhibitors, slow dissociating intermediates, transtion state analogues
Covalent intermediates which take time to dissociate from the enzyme
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
More likely to be toxic and exhibit serious side effects. It may be a poison.
More likely to be toxic and exhibit serious side effects. It may be a poison.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Covalent intermediates which take time to dissociate from the enzyme.
A measure of drug/substance activity expressed in terms of the amount required to produce an effect of given intensity.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group.
Nonspecific of certain types of enzymes
>90% inhibition before any increase in substrate concentration occurs.
Specific or non specific
Potent enough, dose is mg - grams.
Potency and specificity:
Potent enough, dose is mg - grams.
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
To do this an enzyme needs to be ~x20 the Ki value.
Potent enough to work in vivo
Higher affinity than substrate => potent enough to work in vivo at reasonable concentrations.
Covalent intermediates which take time to dissociate from the enzyme
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
High affinity complexes with the Enz and may have Ki values in the order of nanomolar (10-9 mol L-1 ).
A simple reversible inhibitor
Binds noncovalently (ionic interactions, hydrogen bonds, H-bonds) to the enzyme
Competitive inhibitors which are conformationally restricted and/or have many noncovalent interactions leading to long lasting complexes.
Conformationally Restricted Competitive Inhibitor
Higher affinity than substrate to enzyme active site
Strength of binding is of a higher order to the substrate I.e. Ki will be different size to Km.
Binds to Enz and decreases the Enz activity instantaneously and reverses within the time of the enzyme action.
Strength of binding is of a similar order to the substrate I.e. Ki will be of similar size to Km.
Binds to allosteric site on enzyme
A simple reversible inhibitor
Binds noncovalently (ionic interactions, hydrogen bonds, H-bonds) to the enzyme
Competitive inhibitors which are conformationally restricted and/or have many noncovalent interactions leading to long lasting complexes.
Conformationally Restricted Competitive Inhibitor
Higher affinity than substrate to enzyme active site
Strength of binding is of a higher order to the substrate I.e. Ki will be different size to Km.
Binds to Enz and decreases the Enz activity instantaneously and reverses within the time of the enzyme action.
Strength of binding is of a similar order to the substrate I.e. Ki will be of similar size to Km.
Binds to allosteric site on enzyme
Tight binding inhibitors
Conformationally Restricted Competitive Inhibitor
Binds to Enz and decreases the Enz activity instantaneously and reverses within the time of the enzyme action.
Competitive inhibitors which are conformationally restricted and/or have many noncovalent interactions leading to long lasting complexes.
Binds noncovalently (ionic interactions, hydrogen bonds, H-bonds) to the enzyme
Binds to active site on enzyme in competition with substrate
Quasi-irreversible inhibitor
Tight binding inhibitors
Conformationally Restricted Competitive Inhibitor
Binds to Enz and decreases the Enz activity instantaneously and reverses within the time of the enzyme action.
Competitive inhibitors which are conformationally restricted and/or have many noncovalent interactions leading to long lasting complexes.
Binds noncovalently (ionic interactions, hydrogen bonds, H-bonds) to the enzyme
Binds to active site on enzyme in competition with substrate
Quasi-irreversible inhibitor
Analogues of a transition state (or reaction intermediate) for the enzyme catalysed reaction.
nonspecific enzyme inhibitor
Poison
Quasi irreversible inhibitors
simple reversible inhibitor
Tight Binding Inhibitors
Conformationally Restricted Competitive Inhibitor
Slowly Dissociating Intermediate
Transition state analogues
Analogues of a transition state (or reaction intermediate) for the enzyme catalysed reaction.
nonspecific enzyme inhibitor
Poison
Quasi irreversible inhibitors
simple reversible inhibitor
Tight Binding Inhibitors
Conformationally Restricted Competitive Inhibitor
Slowly Dissociating Intermediate
Transition state analogues
Agonist
Drugs bind to receptors, often with high affinity, but do not activate the receptor so they have no intrinsic efficacy.
Rate constants for receptor activation and deactivation, respectively
Measure of the affinity of the drug for the receptor
Stabilise the receptor in the R * state.
Exhibit constitutive activity. In other words, the inactive R complex can convert to the active R * state in the absence of an agonist.
Agonists capable of binding to and stabilising the receptor in both the R and R * states such that although there is full occupancy of the receptor, the efficacy is lower because there are fewer receptors available for conversion to the active state.
A drug that binds to its receptor such that drug-receptor complex is more likely to be in the active R * conformation rather than the inactive R state
always given in molar (moles L−1 ) units and we can use this as a measure of drug POTENCY.
KA (koff / kon)
Stabilise the receptor in the R * state.
A drug that binds to its receptor such that drug-receptor complex is more likely to be in the active R * conformation rather than the inactive R state
Agonists capable of binding to and stabilising the receptor in both the R and R * states such that although there is full occupancy of the receptor, the efficacy is lower because there are fewer receptors available for conversion to the active state.
maximum response achievable from an applied or dosed agent.
Rate constants for receptor activation and deactivation, respectively
Measure of the affinity of the drug for the receptor
always given in molar (moles L−1 ) units and we can use this as a measure of drug POTENCY.
Efficacy
maximum response achievable from an applied or dosed agent.
Measure of the affinity of the drug for the receptor.
Rate constants for receptor activation and deactivation, respectively
Agonists capable of binding to and stabilising the receptor in both the R and R * states such that although there is full occupancy of the receptor, the efficacy is lower because there are fewer receptors available for conversion to the active state.
bonds formed are relatively weak (hydrogen bonds or van der Waals, for example). Very few involve covalent bonds which are essentially irreversible.
prevent an agonist from binding to the receptor and activating it.
Positive intrinsic activity
No intrinsic activity
kα and kβ
rate constants for receptor activation and deactivation, respectively.
Dissociation constants for drug receptor complex
Agonists capable of binding to and stabilising the receptor in both the R and R * states such that although there is full occupancy of the receptor, the efficacy is lower because there are fewer receptors available for conversion to the active state.
Drug affinity function
Drug potency function
Drug efficacy function
Partial agonists
Exhibit constitutive activity. In other words, the inactive R complex can convert to the active R * state in the absence of an agonist.
Agonists capable of binding to and stabilising the receptor in both the R and R * states such that although there is full occupancy of the receptor, the efficacy is lower because there are fewer receptors available for conversion to the active state.
Drugs bind to receptors, often with high affinity, but do not activate the receptor so they have no intrinsic efficacy.
Will act as an antagonist in the presence of a full agonist because it occupies sites on the receptor thus preventing it from being converted to the R * state.
A drug that binds to its receptor such that drug-receptor complex is more likely to be in the active R * conformation rather than the inactive R state
Inverse agonists
Exhibit constitutive activity. In other words, the inactive R complex can convert to the active R * state in the absence of an agonist.
A drug that binds to its receptor such that drug-receptor complex is more likely to be in the active R * conformation rather than the inactive R state
Agonists capable of binding to & stabilising receptor in both the R and R * states such that although there is full occupancy of receptor, the efficacy is lower because there are fewer receptors available for conversion to active state.
Inverse drug receptor complex from DR active state to DR inactive state
prevent an agonist from binding to the receptor and activating it.
Will act as an antagonist in the presence of a full agonist because it occupies sites on the receptor thus preventing it from being converted to the R * state.
Negative intrinsic activity
Bind to receptors, often with high affinity, but do not activate the receptor so they have no intrinsic efficacy.
Constituitive activity
Can be competitive or noncompetitive
A receptor which is capable of producing a biological-response in the presence of a bound-ligand is said to display
Drugs bind to receptors, often with high affinity, but do not activate the receptor so they have no intrinsic efficacy.
Displayed by receptors in neuromuscular junction
Displayed by inverse agonists
A receptor which is capable of producing a biological-response in the absence of a bound-ligand is said to display
Antagonists
A drug that binds to its receptor such that drug-receptor complex is more likely to be in the active R * conformation rather than the inactive R state
Activity can be competitive or noncompetitive
Agonists capable of binding to & stabilising receptor in both the R and R * states such that although there is full occupancy of receptor, the efficacy is lower because there are fewer receptors available for conversion to active state.
Inactive R complex can convert to active R * state in the absence of an agonist. Drugs that bind to the R complex now have the effect of reducing the response mediated by constitutively active receptors.
Drugs bind to receptors, often with high affinity, but do not activate the receptor so they have no intrinsic efficacy.
prevent an agonist from binding to the receptor and activating it.
Competitive antagonist
Competitive antagonism
Binds to rec site distinct from agonist or to element in cascade of events leading to biological respones. Not reversible/effect overcome by ^[agonist]
Competitively binds to active site of agonist and overcome by ^[agonist]
Non competitive antagonist
Competitive antagonism
Binds to rec site distinct from agonist or to element in cascade of events leading to biological respones. Not reversible/effect overcome by ^[agonist]
Competitively binds to active site of agonist and overcome by ^[agonist]
May appear irreversible as dissociation may occur slowly
Competitive antagonism
Binds to rec site distinct from agonist or to element in cascade of events leading to biological respones. Not reversible/effect overcome by ^[agonist]
Competitively binds to active site of agonist and overcome by ^[agonist]
Competitive antagonism
Binds to rec site distinct from agonist or to element in cascade of events leading to biological respones. Not reversible/effect overcome by ^[agonist]
Competitively binds to active site of agonist and overcome by ^[agonist]
Explain the basic principles of drug-receptor binding and be familiar with the terms efficacy and potency.
Define the terms full agonist, partial agonist, competitive and non-competitive antagonist and be able to represent their effects graphically.
{"name":"BIO PHAY1003", "url":"https://www.quiz-maker.com/QPREVIEW","txt":"Outline cell theory, size of viruses, What are viruses\/virions...","img":"https://cdn.poll-maker.com/8-390370/ppi.png?sz=1200-056037026053"}
More Quizzes
Are you staying dry?
100
Sabby's vibe check
7423
Your opinions about the Life Cover
100
Inventory Management SOP Quiz: Boot Inventory
520
Which of the Following Is a Subordinate Clause? Challenge
201026122
Free Biology Final Review
201021828
Test Your Knowledge: India's 5 Traits of Civilization
201053928
Take the Free Milliseconds Test - Time Conversion Challenge
201052648
Ultimate Drama: Test Stage Presence & Theatre Skills
201030216
Which Super Mario Galaxy Character Are You? Find Out Now
201032092
Test Your Medical Terminology: The Nervous System
201032501
Free Factoring Polynomials Practice: Test Your Skills
201024747