Chem 110 study guide
Dalton's Atomic Theory states........
That an atom is predominantly empty space.
That energy is neither created nor destroyed during a chemical reaction.
That all elements have several possible isotopes.
That matter is composed of small, indestructible particles.
A substance that can't be chemically broken down into other stable substances is called ..........
Heterogeneous matter.
Homogeneous matter.
An element.
A compound
Two or more substances in a mixture where the composition varies to the naked eye is referred to as........
A homogeneous mixture.
A compound.
A solution.
A heterogeneous mixture.
A physical change occurs when......
Propane is burned to produce heat.
Glucose is converted into energy in your cells.
Salt is dissolved in water.
Iron rusts.
A piece of metal has a density of 1.58 g/mL. When a student places the metal in a graduated cylinder the volume of liquid in the cylinder rises from 21.25 mL to 26.47 mL. What is the mass of the metal? D = m/V
5.22 g
12.44 g
16.51g
8.25 g
The outside air temperature is 40oF. What is that temperature in kelvin? K = oC + 273 T(°C) = (T(°F) - 32) / 1.8
277
377
291
313
How many significant figures are in 0.00523980?
4
7
6
5
The recommended adult dose of Elixophyllin, a medicine used to treat asthma, is 6.00 mg/Kg of body mass. Calculate the dose in mg for a 115 lb person. 1 lb = 453.59 g 1 Kg = 1000 g 1g = 1000mg
24 mg
313 mg
1521 mg
1.5 mg
Identify the best description of an atom.
Protons and neutrons in the nucleus with electrons in orbitals around it.
Electrons and neutrons in the nucleus with protons in orbitals around it.
Protons in the nucleus with neutrons and electrons in orbitals around it.
Protons and electrons in the nucleus with neutrons in orbitals around it.
The mass number "A" is equal to......
The sum of the number of protons and electrons in the atom.
The sum of the number of protons, electrons and neutrons in the atom.
The weight averaged molecular weight (in AMU's) of the atom.
The sum of the number of protons and neutrons in the nucleus.
Which of the following statements about isotopes is true?
If given the Atomic Number of an element, an isotope is defined by the mass number of that element.
Isotopes of the same element generally have about the same mass.
Electrons and neutrons have about the same mass.
Isotopes of the same element differ only in the number of electrons they contain in their outer orbital.
Ions differ from the parent element in the number of.......?
Neutrons
Protons and electrons
Protons
Electrons
Which of the following does not describe a metal?
Tends to form anions
Tends to form compounds with non-metals
Good conductor of electricity
found on the left side of the periodic table
Calculate the atomic mass of element X if element X has 2 isotopes with the following masses and abundances: Isotope X-1 106.90509 51.84% Isotope X-2 108.90476 48.46%
108.00
107.9
108.32
108.19
What is the mass of 2.63 moles of nickel? (MW of Ni 58.69)
0.129 g
356 g
22.3 g
154 g
Identify a cation.
An element that has gained an electron
An element that has lost a proton
An element that has lost an electron
An element hat has lost a neutron
An ionic bond is best described as .........
The attraction between two metal atoms
The transfer of electrons from one atom to another
The sharing of electrons between atoms
The attraction between two non-metal atoms
Give the name for SnO. (Sn = tin, Oxide ion is -2)
Tin (II) oxide
Tin oxide
Tin (IV) oxide
Tin (III) oxide
Give the correct formula for aluminum sulfate. (Aluminum ion is Al+3, Sulfate ion is SO4-2)
Al3(SO4)2
Al2(SO4)3
Al(SO4)3
Al2SO4
Calculate the formula mass of Al(C2H3O2)3. (Al mass is 26.98, C mass is 12.01, H mass is 1.008, O mass is 16)
258.09
139.99
204.13
86.03
What is the mass percent composition of sulfur in Al2(SO4)3 (Al mass is 26.98, S mass is 32.1, O mass is 16)
42.73%
21.38%
35.97%
28.12%
Determine the molecular formula for a compound with a molecular weight of 92.0 g/mol and an empirical formula of NO2. (N mass 14.00, O mass 16.0)
N2O3
N3O6
N2O5
N2O4
What type of bonding is found in the compound NF3?
Metallic bonding
Hydrogen bonding
Ionic bonding
Covalent bonding
Which of the following exists as a diatomic molecule?
Phosphorous
Krypton
Lithium
Bromine
Balance the following equation: _____CH4 + _____Cl2 -----> _____CCl4 + _____HCl What is the coefficient in front of the HCl?
2
5
3
4
Which of the following substances best describes the particulate nature of matter?
Water molecule
Rock
Grain of sand
Bacterium
Which of the following substances is heterogeneous
Pizza
Water with salt dissolved in it
Jello
Homogenized milk
If you add solid ammonium chloride to a beaker of water the water will get cold. Therefore the reaction of ammonium chloride with water must be......
Endothermic
Exothermic
Which of the following would be classified as a chemical change?
Making toast
Dissolving salt in water
Adding vinegar to olive oil and shaking it real hard
Making peanut butter from peanuts
How many significant figures are in 0.0007770?
4
3
7
8
What type of substance cannot be chemically decomposed into other stable pure substances?
An element
A molecule
Homogeneous matter
A compound
Which of the following best describes a homogeneous sample?
Uniform appearance and composition throughout
It is always a pure substance
Visibly different parts or phases
A mixture of diamond and graphite, which are both forms of carbon
Which of the following statements is true?
The number of neutrons always equals the number of protons in an element
The total contribution to the mass of an atom from protons and electrons varies among the atoms of that element due to the existence of isotopes.
Given the atomic number of an element, the atomic mass of that element can be calculated.
None of the above
Which of the following masses is the closest to one atomic mass unit (AMU)?
1/12 the mass of one 12C atom
1.66 g
12 mg
12 g
Which of the following statements is incorrect?
An element is identified by its mass number "A".
The atomic number is equal to the number of protons in the nucleus.
An element is identified by its atomic number "Z".
The mass number is equal to the number of protons and neutrons in the nucleus.
In a chemical reaction, matter is neither created or destroyed. To which Law does this refer?
Law of Definite Proportions
Law of Conservation of Mass
First Law of Thermodynamics
Law of Modern Atomic Theory
All samples of a given compound, regardless of their source or how they were prepared, have the same ratios of their constituent elements. To which Law does this refer?
Law of Definite Proportions
Law of Conservation of Mass
First Law of Thermodynamics
Law of Multiple Proportions
When two elements form two different compounds the mass of element B that combine with one gram of element A can be expressed as a ratio of small whole numbers. To which Law does this refer?
Law of Modern Atomic Theory
First Law of Thermodynamics
Law of Multiple Proportions
Law of Definite Proportions
Which of the following is an example of the Law of Multiple Proportions?
Two different compounds formed from carbon and oxygen have the following mass ratios: 1.33g O: 1g C, and 2.66g of O: 1 g C
Two different samples of table salt are found to have the same ratio of sodium to chlorine.
A sample of chlorine is found to contain three times as much Cl-35 as Cl-37.
Nitrogen Dioxide always has a mass ratio of 2.28g O: 1g N
Which of the following statements is FALSE according to Dalton's Atomic Theory?
All atoms of chlorine have identical properties that distinguish them from other elements.
An atom of nitrogen can be broken down into smaller particles that will still have the unique properties of nitrogen.
One carbon atom will combine with one oxygen atom to form a molecule of carbon monoxide.
Atoms combine in simple whole number ratios to form compounds.
Identify the description of an atom.
Electrons and Neutrons in the nucleus, protons in orbitals.
Electrons in the nucleus, protons and neutrons in orbitals.
Protons and electrons in the nucleus, neutrons in orbitals.
Protons and Neutrons in the nucleus, electrons in orbitals.
Identify the charges of protons, neutrons and electrons.
Protons +1, Electrons -1, Neutrons 0
Protons +1, Electrons 0, Neutrons -1
Protons -1, Electrons +1, neutrons 0
Protons 0, Electrons -1, neutrons +1
The mass number equals....
The sum of electrons and protons.
The sum of the protons and neutrons in the nucleus.
The sum of neutrons and electrons.
The sum of the protons, neutrons and electrons
Which of the following statements about sub-atomic particles is TRUE?
A neutral atom contains the same number of electrons as protons.
Protons and neutrons have opposite, but equal in magnitude, charges.
Electrons make up most of the mass of the atom.
Protons have about the same mass as electrons.
Which of the following statements about isotopes is TRUE?
Isotopes of the same element have the same mass.
Isotopes of the same element usually have different properties.
Isotopes of the same element differ only in the number of electrons they contain.
Some elements have three or more naturally occurring isotopes .
An ionic bond is best described as.....
The transfer of electrons from one atom to another
The sharing of electrons
The attraction that hold atoms together in a polyatomic ion
The attraction between two metal atoms
A covalent bond is best described as......
A sharing of electrons
A bond between a metal and a non-metal
The transfer of electrons from one atom to another
A bond between two polyatomic ions
What is the name for SnO?
Tin (II) oxide
Tin (I) oxide
Tin (III) oxide
Tin (IV) oxide
What is the formula for sodium perchlorate?
NaClO4
NaClO
NaClO2
NaClO3
What is the name for H2SO4?
Sulfuric acid
Sulfurous acid
Persulfuric acid
Hyposulfuric acid
Calculate the molar mass of Mg(ClO4)2.
223.21
247.52
123.76
75.76
How many millimoles of Ca(NO3)2 contain 4.78 x 1022 formula units of Ca(NO3)2? The molar mass of Ca(NO3)2 is 164.10 g/mol.
79.4 mmol Ca(NO3)2
57.0 mmol Ca(NO3)2
20.7 mmol Ca(NO3)2
13.0 mmol Ca(NO3)2
What is the mass (in kg) of 6.89 x 1025 molecules of CO2? The molar mass of CO2 is 44.01 g/mol
5.04 kg
3.85 kg
6.39 kg
2.60 kg
Calculate the mass percent composition of sulfur in Al2(SO4)3
28.12%
21.38%
35.97%
9.372%
Determine the molecular formula of a compound that has a molar mass of 92.0 g/mol and an empirical formula of NO2
N2O4
N2O3
N2O5
N3O6
How many grams of Li3N can be formed from 1.75 moles of lithium as per the following reaction sequence? Assume an excess of nitrogen. 6Li(s) + N2(g) ----------------> 3Li3N(s) MW Li 6.94 g/mol MW N 14.0 g/mol
29.6 g
20.3 g
18.3 g
15.1 g
What is the percent yield when 28.16 g of CO2 is formed by the reaction of 4.00 moles of C8H18 with 4.00 moles of O2? 2C8H18(g) + 25O2(g) -------> 16CO2(g) + 18H2O
20.0%
50.0%
25.0%
12.5%
Determine the molarity of a solution formed by dissolving 97.7 g of LiBr in enough water to yield 750 mL. MW Li 6.94g/mol, MW Br 79.9g/mol
0.130M
1.50M
0.768M
1.18M
Identify HCl(aq).
Weak acid, strong electrolyte
Weak acid, weak electrolyte
Strong acid, strong electrolyte
Strong acid, weak electrolyte
Choose the true statement.
A strong electrolyte is comprised of very reactive molecules.
A weak electrolyte is comprised of inert molecules.
A neutral molecular compound dissolved in water is considered a strong electrolyte.
A weak acid solution consists mostly of unionized acid molecules.
Which element is being oxidized (if any) in the following reaction? CH4(g) + 2O2(g) -------> CO2(g) + 2H2O(g)
H
O
None of the above
C
Automotive air bags inflate when sodium azide explosively decomposes into its constituent elements. How many moles of nitrogen gas is evolved from the decomposition of 2.88 mol of NaN3 (sodium azide)? 2NaN3(s) ----------> 3N2(g) + 2Na(s)
8.64
4.32
1.44
1.92
Balance the chemical equation given below and determine the number of moles of iodine that reacts with 30.0 g of aluminum. __________Al(s) + _________I2(s) -----------> _______Al2I6(s)
1.67 moles
2.22 moles
0.741 moles
3.33 moles
Which substance is the limiting reagent when 2.0 g of sulfur reacts with 3.0 g of oxygen and 4.0 g of sodium hydroxide by the following chemical reaction: 2S(s) + 3O2(g) + 4NaOH(aq) ---------> 2Na2SO4(aq) + 2H2O(l)
NaOH
Sulfure
Oxygen
None of the above
Determine the concentration of a solution prepared by diluting 20.0 mL of a 0.20M solution of CsCl to 250mL.
0.0160M
0.0320M
0.0080M
0.160M
An instrument used to measure the pressure of a gas in the laboratory is called a ________?
Manometer
Spectrometer
Sphygmomanometer
Barometer
The volume of a gas is directly proportional to the temperature of a gas is known as _________________
Avagadro's law
Dalton's Law
Boyles' Law
Charles' law
The volume of a gas is inversely proportional to the pressure of a gas is known as ______?
Boyles' law
Charles' Law
Dalton's Law
Avogadro's Law
If a sample of 0.29 moles of Ar occupies 3.8L under standard conditions what volume will 0.66 moles occupy under the same conditions?
5.0 L
17 L
12 L
8.6 L
To what temperature must a balloon, initially at 25oC and 2.00 L, be heated in order to have a volume of 6.00 L?
655 K
403 K
894 K
993 K
What pressure will 14.0 g of CO exert in a 3.5 L container at 75 C?
4.1 ATM
6.4 ATM
2.3 ATM
5.0 ATM
Which of the following will cause the volume of an ideal gas to triple in value?
Raising the absolute temperature by a factor of three while increasing the pressure by a factor of 3.
Lowering the absolute temperature by a factor of 3 while increasing the pressure by a factor of 3.
Lowering the absolute temperature by a factor of 3 at constant pressure.
Lowering the pressure by a factor of three and holding the temperature constant.
Determine the density of CO2 gas at STP.
4.46 g/L
1.96 g/L
1.80 g/L
2.24 g/L
The total pressure of a gas mixture is equal to the sum of the partial pressures of its components is known as ____?
Dalton's Law
Ideal Gas Law
Avogadro's Law
Charles' Law
A mixture of 1.0 moles of H2 and 1.0 moles of Ne are at STP in a rigid container. Which of the following statements about these gases is false?
They both have he same average kinetic energy.
They have different molecular speeds.
They both contribute the same mass to the mixture.
They both have the same number of particles.
Energy that is associated with the position or composition of an object is called____________.
Thermal energy
Potential energy
Chemical energy
Kinetic energy
A sample of copper absorbs 43.6J of heat, resulting in a temperature rise of 75oC. Calculate the mass of the copper sample if the specific heat capacity of copper is 0.385J/goC. Q = m X c X deltaT
3.64 g
1.26 g
1.51 g
6.62 g
Which statement is False?
Delta Hrxn is a measure of heat of reaction.
An endothermic reaction has a positive deltaH.
An exothermic reaction gives off heat to the surroundings.
Delta Erxn is a measure of heat.
Using the following equation for the combustion of octane, calculate the number of moles of carbon dioxide formed from 100.0 g of octane. The molar mass of octane is 114.33 g/mol and the molar mass of carbon dioxide is 44.0095 g/mol. 2C8H18 + 25O2 -----> 16CO2 + 18H2O DeltaHorxn = -11018 KJ DeltaHo = -5509 KJ/mol
8.00 moles
6.997 moles
18.18 moles
10.92 moles
Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter. The reaction causes the temperature of the resulting solution to fall below room temperature. Which of the following statements is True?
Energy is leaving the system during the reaction
None of the statements are true.
The products have a lower potential energy than the reactants.
The reaction is exothermic.
A piece of iron (mass = 25.0 g) at 125oC is placed in a coffee cup containing 25.0 g of water at 25oC. What will the final temperature of the water be? The specific heat capacity of iron is 0.449 J/goC and water is 4.18 J/goC.
75degC
52degC
14degC
35degC
Which of the following is not a standard state?
For a liquid it is 25degF.
For a gas it is 1 atm.
For a solid it is 25degC.
For a solution it is 1M.
Which of the following is True if the DeltaEsys = -115J?
None of the above are True.
The system is gaining 115J while the surroundings are losing 115J.
Both the system and the surroundings are losing 115J.
The system is losing 115J while the surroundings are gaining 115J.
Define specific heat capacity.
The quantity of heat required to raise the temperature of 1 g of a substance 1degC.
The quantity of heat required to raise the temperature of 1 mole of a substance 1degC.
The quantity of heat required to raise the temperature of 1 g of a substance 1degF
The quantity of heat required to lower the temperature of 1 L of a substance 1degC
Which of the following processes is endothermic?
A hot cup of coffee gets cool.
The combustion of butane.
The freezing of water.
The vaporization of rubbing alcohol.
The vertical height of a wave is called ________________
Ampltiude
Frequency
Median
Wavelength
The number of cycles that pass through a stationary point is called__________________
Frequency
Wavelength
Amplitude
Median
If the energy of a photon increases________
The speed increases
The wavelength increases
The frequency decreases
The frequency increases
Which of the following is true?
The uncertainty principle states that we can never know the exact position and speed of an electron.
All are true
The emission spectrum of a particular element is specific for that element and can be used to identify that element.
An orbital is the volume in which we are most likely to find the electron.
For n=3 what are the possible sublevels?
S
S,p
S,p,d
S,p,d,f
Which of the following quantum numbers describe the size and energy of an orbital?
Angular momentum quantum number
Principle quantum number
Spin quantum number
Magnetic quantum number
Describe the shape of a p orbital.
Spherical shaped
Dumbbell-shaped
Three attached balls
Four attached balls
What is the maximum number of p orbitals possible?
1
3
2
4
Describe the shape of an s orbital.
Four attached balls
Three attached balls
Dumbbell-shaped
Spherical
Quantum theory is based on the concept that _____________
Sunlight shining through a prism is split into discrete wavelengths
The electron in a Bohr hydrogen atom can only be found in its excited state
Electron energies are restricted to certain distinct values
The ground state energy of an electron is dependent upon the temperature of the material
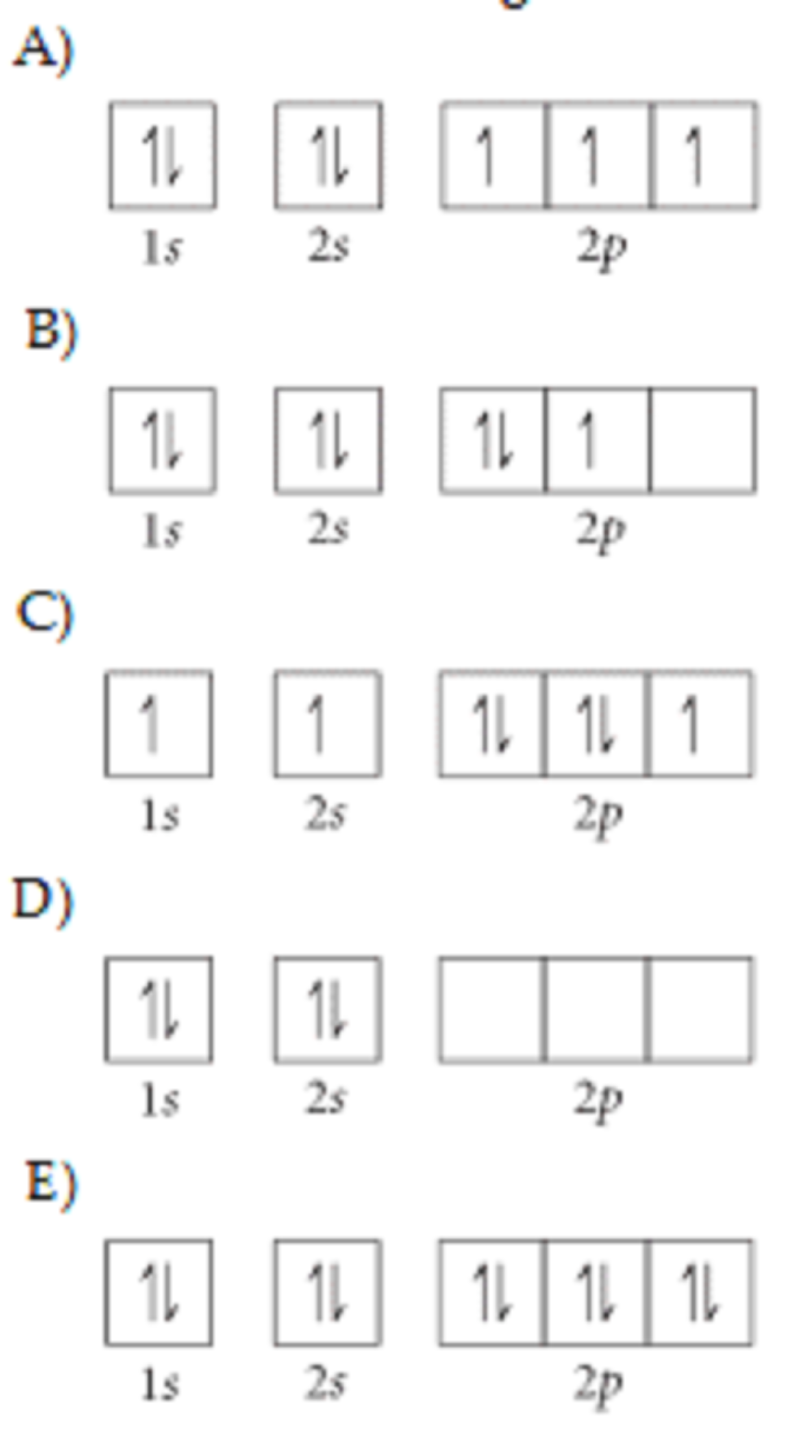
Choose the orbital diagram that represents the ground state of nitrogen.
A
B
C
D
No two electrons can have the same set of quantum numbers is known as _______
Hund's rule
Pauli Exclusion Principle
Heisenberg Uncertainty Principle
Aufbau Principle
Which of the following statements is false?
An orbital that penetrates into the region occupied by the core electrons is less shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy.
Two electrons in the same orbital can have the same spin.
It is not possible for two electrons in the same atom to have identical values for all four quantum numbers
All of the above are true
The element that corresponds to the electron configuration 1s22s22p63s23p64s2 is______
Calcium
Chromium
Iron
Potassium
What is the number of valence electrons for argon?
2
4
6
8
How many valence electrons do the alkaline earth metals possess?
2
4
6
8
Place the following elements in order of increasing atomic radius….. As O Br
As
Br
As
O
Describe the reaction of noble gases with alkali metals.
Vigorous
Mild
Inert
Exothermic
Halogens can react with each other to form ______
Metal halides
Covalent bonds
Salts
Ionic bonds
How many of the following elements have one unpaired electron in their ground state? B Al O F
1
2
3
4
A sample of calcium fluoride was decomposed into its constituent elements and produced 0.294 g of fluorine. How many g of calcium were formed? HINT: Write balanced equation for: calcium fluoride --> calcium metal + fluorine gas. MW calcium 40.08 g/mol MW fluorine 19 g/mol Calcium ion Ca +2 Fluoride ion F-1
280 g
3.13 g
0.280 g
0.309 g
A 14.01 g sample of N2 reacts with 3.03 g of H2 to form ammonia (NH3). If ammonia is the only product what mass of ammonia is formed? MW N 14 g/mole MW H 1.008 g/mol Hint: Write out balanced reaction first.
3.02 g
17.01 g
14.01 g
23.07 g
Consider the following reaction. How many grams of water are required to form 75.9 g of HNO3? Assume that there is an excess of NO2 present. The molar masses are as follows: H2O = 18.02 g/mol, HNO3 = 63.02 g/mol Remember: When you see grams, convert to moles. 3NO2 (g) + H2O(l) --> 2HNO3(aq) + NO(g)
26.5 g
21.7 g
38 g
10.9 g
Consider the following reaction. How many moles of CO2 are formed from the reaction of 4.0 moles of C8H18 and 4.0 moles of O2? MW C8H18 = 114 g/mol CO2 = 44 g/mol O2 = 32 g/mol Hint: find limiting reagent. 2C8H18 + 25O2 --> 16CO2 + 18H2O
64.0 moles
16.0 moles
2.56 moles
0.640 moles
Determine the percent yield of a reaction that produces 28.65 g of Fe when 50.0 g of Fe2O3 react with excess Al according to the following reaction: Fe2O3(s) + 2Al(s) --> Al2O3(s) + 2Fe(s) MW Fe2O3 159.7 g/mol Fe 55.85 g/mol Percent yield = actual yield/theoretical yield
57.3%
61.03%
81.93%
20.02%
Determine the molarity of a solution formed by dissolving 97.7 g of LiBr in water with a total volume of 750 mL. MW LiBr = 86.85 g/mol Remember: Molarity = moles/L
1.18 M
0.768 M
2.30 M
1.50 M
Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of H2SO4 (an acid) and KOH (a base) are mixed.
H+(aq) + OH-(aq) + 2K+(aq) + SO4-2(aq) ---> H2O(l) + K2SO4(s)
2K+(aq) + SO4-2(aq) --> K2SO4(s)
H+(aq) + OH-(aq) --> H2O(l)
H2 2+(aq) + OH-(aq) ---> H2(OH)2(l)
Which of the following statements is true? An electrolyte conducts electricity. An electrolyte is a solution of ethanol in water. An electrolyte occurs when sugar is dissolved in water. All of the above statements about electrolytes are true.
An electrolyte conducts electricity.
An electrolyte is a solution of ethanol in water.
An electrolyte occurs when sugar is dissolved in water.
All of the above statements about electrolytes are true.
How many L of H2 at STP are required to produce 0.965 g of H2O by the following reaction? Assume excess oxygen. 2H2(g) + O2(g)----> 2H2O(g) STP = 273K, 1 atm, 22.4L/mol Remember: Convert g to moles.
1.24 L
3.31 L
0.78 L
2.14 L
Define diffusion.
The process whereby gas particles increase in speed with a rise in temperature.
The process whereby gas particles move from an area of high density to low density.
The process whereby gas particles move through a small opening between areas of high density to low density.
The process whereby gas particles move from an area of low density to an area of high density.
How much energy (in J) is transferred from the system to the surroundings when 475 g of water is cooled from 95oC to 14oC. cH2O = 4.184J/g Q = m x c x deltaT
1.6 KJ
16 KJ
0.16 KJ
160 KJ
A syringe contains 0.65 moles of He gas that occupies 750mL. What volume (in L) of gas will the syringe hold if 0.35 moles of Ne were added? Remember: All gases can be regarded as identical. Hint: n and V are directly proportional. Pressure and temperature are constant.
2.1 L
1.2 L
0.87 L
1.9 L
To what temperature must a balloon, initially at 25oC and 2.0 L, be heated to have a new volume of 6.0 L? Assume P is constant. 25 deg C = 298 K V1/T1 = V2/T2
655 K
894 K
75 K
403 K
What is the pressure in a 3.5 L closed vessel containing 14.0 g of CO gas at 75oC? MW CO = 28 g/mole R = 0.0802 PV = nRT Convert T to kelvin.
6.4 ATM
2.3 ATM
3.98 ATM
5.0 ATM
A 0.341 g sample of an unknown halogen gas occupies 109 mL at 398 K and 1.41 atm. What is the identity of the gas? MW F2 38 g/mol Cl2 70.9 g/mol Br2 159.8 g/mol I2 253.8 g/mol HINT: PV = nRT, R = 0.0802, solve for n (# of moles) then calculate MW (g/mol).
I2
Cl2
Br2
F2
A mixture of 10.0 g of Ne and 10.0 g of Ar have a total pressure of 1.6 atm. What is the partial pressure due to Ne? Ne = 20.18 g/mol Ar = 39.95 g/mol HINT: Partial pressures are based on mole% not weight% so calculate moles of each.
0.54 atm
1.3 atm
1.1 atm
0.80 atm
Energy that is associated with position or composition of an object is called _____________
Thermal energy
Chemical energy
Potential energy
Kinetic energy
According to the following thermochemical equation what mass of HF (in g) is required to produce 345 KJ of energy. SiO2(s) + 4HF ---> SiH4(g) + 2H2O(l) deltaH = -46 KJ/mol MW HF 20.1 g/mol HINT: How many times does 46 KJ go into 345 KJ?
107 g
42.7 g
150 g
173 g
Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter. The reaction causes the temperature of the resulting solution to fall below room temperature. Which of the following statements is true?
Energy is leaving the system during the reaction.
The products have a lower potential energy than the reactants.
The reaction is exothermic.
None of the above are true.
Given the reaction below: N2(g) + 3H2(g) -----> 2NH3(g) deltaH = -92.6 KJ What is the value of deltaH for the following reaction? 2NH3(g) -----> N2(g) + 3H2(g)
92.6 KJ
-92.6 KJ
185.2 KJ
-185.2 KJ
Lead, water, sulfur, and arsenic have specific heats of 0.128, 4.18, 0.706, 0.329 J/goC, respectively. Which of these would require the smallest amount of heat to increase its temperature by 10oC (assume all samples have the same mass). Specific heat is defined as the amount of heat necessary to raise the temperature of 1 g of a substance 1 deg C.
Sulfur
Lead
Arsenic
Water
Suppose you have 125 mL of coffee in a well-insulated cup, but the coffee is at 92oC, which is too hot to drink. What volume of cold milk at 12oC would you have to add to reduce the temperature of the coffee to 72oC? Q = m x c x deltaT HINT: The heat lost by the coffee must be gained by the milk so -Qcoffee = Qmilk *** Assume coffee and milk have the same specific heat and density as water: cH2O = 4.184 J/(g oC); dH2O = 1 g/ml
125 mL
88.1 mL
28.5 mL
41.6 mL
Which of the following quantum numbers describes the energy and size of an electron’s orbital?
Spin quantum number
Magnetic quantum number
Principle quantum number
Angular momentum quantum number
Which of the following statements is false? -An orbital is the volume in which the electron is likely to be located. -Part of the Bohr Model proposes that electrons in the hydrogen atom are located in specific orbits around the nucleus. -The Uncertainty Principle states that we can never know both the exact location and speed of an electron. -None of the above is false.
Part of the Bohr Model proposes that electrons in the hydrogen atom are located in particular orbits around the nucleus.
An orbital is the volume in which the electron is likely to be located.
The Uncertainty Principle states that we can never know both the exact location and speed of an electron.
None of the above is false
Which of the following occurs as the energy of a photon increases?
The wavelength increases
The frequency decreases
The wavelength gets shorter
The speed increases
Which of the following statements is True?
A covalent bond has a lower potential energy than the two atoms separately.
A covalent bond is formed by the transfer of electrons from one atom to the other.
Single bonds are shorter than double bonds.
The pair of electrons involved in a covalent bond are referred to as “lone pairs”.
Which of the following is the correct electronic configuration for Ca+2? 1. 1s22s22p63s24p6 2. 1s22s22p63s23p6 3. 1s22s23s23p64s2 4. 1s22s22p63s23p64s2
1
2
3
4
Which of the following is true?
None of these are true.
Ionic compounds at room temperature are good conductors of electricity.
An ionic bond is formed through the sharing of electrons between atoms.
An ionic bond is much stronger than most covalent bonds.
A single covalent bond consists of ___________ of electrons.
0 pairs
1 pair
2 pairs
4 pairs
Identify the number of bonding pairs and lone pairs of electrons in a water molecule.
1 bonding pair, 2 lone pairs
2 bonding pairs, 1 lone pair
2 bonding pairs, 2 lone pairs
1 bonding pair, 1 lone pair
Identify the compound with the smallest dipole moment.
Cl2
LiF
HF
ClF
Using periodic trends, place the following bonds in order of increasing ionic character. Si-P Si-Cl Si-S
Si-Cl
Si-P
Si-P
SI-S
Give the number of valence electrons for CH2Cl2.
16
20
18
22
Identify an ionic bond.
Protons are shared between the two atoms.
Electrons are shared between the two atoms.
Protons are transferred between the two atoms.
Electrons are transferred from one atom to the other.
Which molecule or compound contains a polar covalent bond?
AgI
NCl3
LiF
ZnF
Why is water an extraordinary substance?
Water has an exceptionally high heat capacity.
Water has a low molecular weight and yet it is a liquid at room temperature.
Water has strong intermolecular attraction.
All of the above.
The force between two polar molecules is known as………
Dispersion forces
Ionic forces
Dipole-dipole forces
Hydrogen bonding
What is the strongest type of intermolecular force present in methanol (CH3OH)?
Hydrogen bonding
Dipole-dipole force
Dispersion force
Ion-dipole force
What is the term used to describe the ability of a liquid to flow against gravity up a narrow tube?
Capillary action
Viscosity
Surface tension
Density
Which of the following statements is true?
Intermolecular forces hold the atoms in a molecule together
Vapor pressure increases with temperature
Dispersion forces are generally stronger than dipole-dipole forces
Hydrogen bonds are stronger than covalent bonds
Identify the place with the highest boiling point of water.
Mt. Everest, 29035 feet above sea level
Prescott, Arizona, 5300 feet above sea level
New Orleans, sea level
Death Valley, 282 feet below sea level
Which of the following statements is False?
The rate of vaporization increases with increasing surface area.
The rate of vaporization increases with decreasing intermolecular forces.
Molecules with hydrogen bonding are more volatile than compounds with dipole-dipole attractions.
The rate of vaporization increases with increasing temperature.
How much energy is required to vaporize 98.6 g of ethanol (C2H5OH) at its boiling point if its deltaHvap is 40.5 kJ/mol? BP of water is 100oC.
52.8 kJ
86.7 kJ
18.9 kJ
32.2 kJ
Define sublimation.
A gas become a liquid
A solid becomes a gas
A liquid becomes a gas.
A gas becomes a solid
How much energy is required to heat 36.0 g of water from a liquid at 65oC to a gas at 115oC? The following data may be required: DeltaHvap = 40.7 kJ/mol Cliq = 4.18 J/goC Cgas = 2.01 J/goC Csol = 2.09 J/goC Tmelt = 0oC Tboil = 100oC
10.9 kJ
87.7 kJ
91.7 kJ
63.5 kJ
According to Lewis theory, valence shell electron repulsion theory accounts for .................?
Molecular shape
Valence electrons
Bond lengths
Electronic configuration
Electrons in the highest principle energy level are referred to as..................?
Lone pairs
"f" electrons
Valence electrons
Covalent electrons
True/False Two electrons, both negatively charged, can occupy the same orbital.
True
False
A material with weaker intermolecular attractions will have a _____________vapor pressure.
Impossible to determine
Lower
Higher
Unaffected
How many sublevels are contained in the third principle energy level of a given atom?
18
5
9
3
Which of the following quantum numbers describes the energy and size of an electron’s orbital?
Principle quantum number
Magnetic quantum number
Angular momentum quantum number
Spin quantum number
No two electrons can have the same set of quantum numbers is known as ……………….?
Hund’s Rule
The Pauli Exclusion principle
Aufbau Principle
Heisenberg Uncertainty Principle
Which of the following statements is true?
An orbital that penetrates into the region occupied by core electrons is less shielded from nuclear charge than an orbital that does not penetrate and would therefore have a lower energy.
An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and would therefore have a lower energy.
Two electrons can inhabit the same orbital only if they have the same spin.
None of the above are true.
What element corresponds to the ground state electron configuration of 1s22s22p63s23p64s2?
Strontium
Zinc
Sodium
Calcium
How many valence electrons does aluminum possess?
2
3
5
8
Halogens can react with each other to form…….
Covalent bonds
Ionic bonds
Salts
Metallic bonds
A cation with a +2 charge indicates that the element has……..
Gained 2 protons
Lost 2 electrons
Lost 2 protons
Gained 2 electrons
How many unpaired electrons are present in the ground state of a carbon atom?
1
2
3
4
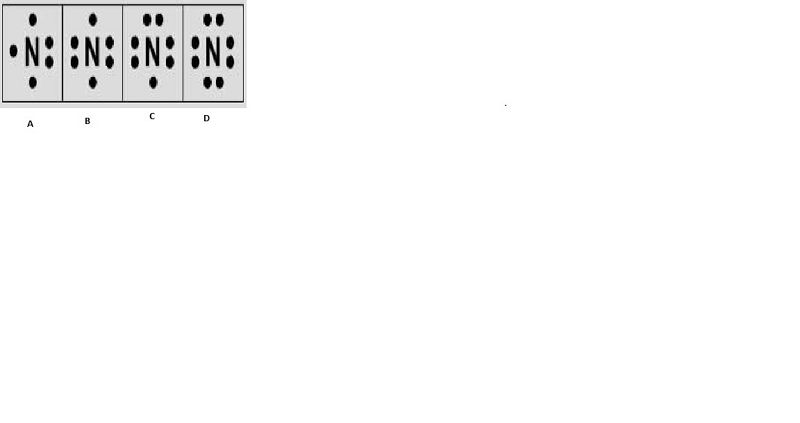
Which of the following is the correct Lewis Dot diagram for nitrogen?
A
B
C
D
Which of the following statements is true?
A covalent bond is generally stronger than an ionic bond.
Ionic solids at room temperature typically conduct electricity.
An ionic bond is generally stronger than a covalent bond.
None of the above are true.
What is the chemical formula for the compound formed from aluminum and oxygen? (Al+3, O-2)
Al3O2
Al2O3
AlO
Al2O
A triple covalent bond contains _____ electrons.
2
3
4
6
Choose the bond that is most polar.
N-O
C-C
C-F
C-N
Place the following elements of decreasing electronegativity............. S F Se
Se>F>S
Se>S>F
S>F>Se
F>S>Se
What is the molecular geometry for CO2?
Linear
Tetrahedral
Trigonal planar
Trigonal pyramid
How many of the following molecules are polar? CO2 H2O CH4 NH3
1
2
3
4
What is the approximate bond angle for a molecule with trigonal planar geometry?
90 degrees
109.5 degrees
120 degrees
180 degrees
Which of the following statements is true? 1. The potential energy of molecules decreases as they get closer to one another. 2. Increasing the pressure on a solid usually causes it to become a liquid. 3. Energy is given off when the attraction between two molecules is broken. 4. Intermolecular forces are generally stronger than bonding forces.
The potential energy of molecules decreases as they get closer to one another.
Intermolecular forces are generally stronger than bonding forces.
Energy is given off when the attraction between two molecules is broken.
Increasing the pressure on a solid usually causes it to become a liquid.
How much energy is required to heat 87.1 g of acetone (MW = 58.08 g/mol) from a solid at -154.0oC to a liquid at -42oC? The following physical data may be necessary: deltaHfus = 7.27 kJ/mol Cliq = 2.16 J/goC Cgas = 1.29 J/goC Csol = 1.65 J/goC Tmelt = -95oC Q = m x C x deltaT Q = m x deltaH
29.4 kJ
18.5 kJ
32.2 kJ
9.97 kJ
Which hypothetical substance has the strongest intermolecular forces? A.) A2X, deltaHvap = 39.6 kJ/mol B.) BY2, deltaHvap = 26.7 kJ/mol C.) C3X2, deltaHvap = 36.4 kJ/mol D.) DX2, deltaHvap = 23.3 kJ/mol
A
B
C
D
{"name":"Chem 110 study guide", "url":"https://www.quiz-maker.com/QPREVIEW","txt":"Dalton's Atomic Theory states........, A substance that can't be chemically broken down into other stable substances is called .........., Two or more substances in a mixture where the composition varies to the naked eye is referred to as........","img":"https://www.quiz-maker.com/3012/CDN/96-4711444/a.png?sz=1200"}
More Quizzes
How Well Do You Know Our Standard French Class?
13623
COLOURS
520
Greek Myth Quiz
210
Alice training
420
Free Planet Earth: Test Your Geology Knowledge Now
201034948
Free Math SAT Practice Test & ACT Questions
201021882
Ultimate Food Webs: Test Your Energy Transfer Skills
201066178
Free Wildflower Identification
201023236
Ultimate Theatre Trivia - Test Your Stage Smarts!
201035449
North & South America Geography: Test Your Skills
201054518
Ultimate Nervous System: Anatomy & Physiology Mastery
201028101
Free Employee Quality Management Knowledge Test
201023421